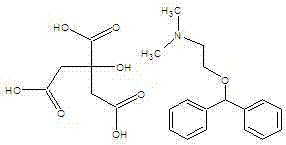
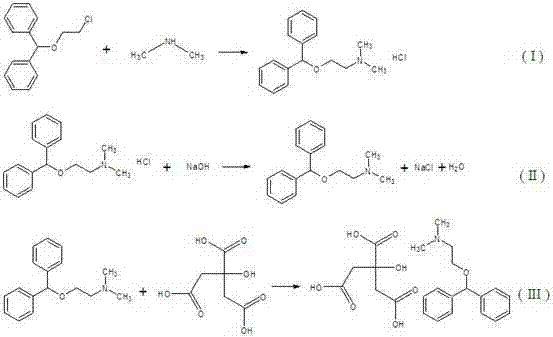
Method for synthesizing citric acid diphenhydramine
A diphenhydramine citrate and synthesis method technology, applied in chemical instruments and methods, carboxylate preparation, organic compound preparation, etc., can solve the problems of cumbersome and complicated operations, low product yields, etc., and achieve simplified operations steps, simplify the purification steps, and shorten the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, the synthesis of bisbenzyl alcohol-beta-chloroethyl ether
[0022] Add 92g of benzhydryl alcohol, 40g of chloroethanol, and 21.8ml of sulfuric acid with a mass fraction of 98% into a three-necked flask (the molar ratio of benzhydryl alcohol, chloroethanol and sulfuric acid is 1:1:0.8), heat up to 70°C and keep the reaction. The reaction process was monitored by thin-layer chromatography. After 6 hours of reaction, the mixture was allowed to stand for stratification, and the oily liquid in the upper layer was collected, washed 3 times with 25% sodium carbonate solution with a mass fraction, and then washed 3 times with hot water at a temperature of 60°C. That is, bisbenzyl alcohol-β-chloroethyl ether was obtained with a yield of 78.5%.
[0023]
Embodiment 2
[0024] Embodiment 2, the synthesis of benzyl alcohol-beta-chloroethyl ether
[0025] Add 92g of benzhydryl alcohol, 52.3g of chloroethanol, and 16.3ml of sulfuric acid with a mass fraction of 98% into a three-necked flask (the molar ratio of benzhydryl alcohol, chloroethanol and sulfuric acid is 1:1.3:0.6), and heat up to 100°C for reaction , monitor the reaction process with thin-layer chromatography, after 4 hours of reaction, let it stand for stratification, collect the oily liquid in the upper layer, wash 3 times with 15% sodium carbonate solution with a mass fraction, and then wash 3 times with hot water at a temperature of 80°C , that is, bisbenzyl alcohol-β-chloroethyl ether was obtained with a yield of 84.3%.
[0026]
Embodiment 3
[0027] Embodiment 3, the synthesis of bisbenzyl alcohol-beta-chloroethyl ether
[0028] Add 92 g of benzhydryl alcohol, 60.4 g of chloroethanol, and 10.9 ml of sulfuric acid with a mass fraction of 98% into a three-necked flask (the molar ratio of benzyl alcohol, chloroethanol and sulfuric acid is 1:1.5:0.4), heat up to 130°C and keep the reaction , monitor the reaction process with thin-layer chromatography, after 3 hours of reaction, let it stand for stratification, collect the oily liquid in the upper layer, wash 3 times with 5% sodium carbonate solution with a mass fraction, and then wash 3 times with hot water at a temperature of 100°C , that is, bisbenzyl alcohol-β-chloroethyl ether was obtained with a yield of 73.6%.
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com