Process for preparing magnesium nitride through airflow method
A technology of magnesium nitride and air flow method, which is applied in the direction of nitrogen-metal/silicon/boron binary compounds, etc., can solve the problems of complex operation of equipment, long reaction time, low yield of magnesium nitride, etc., and achieve uniform product particles, The effect of simple process operation and high synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 (comparative example).
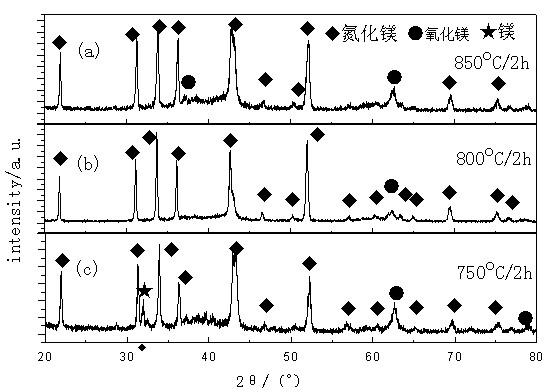
[0023] Put 1.5g of Mg powder in a ceramic boat, put the ceramic boat into the tube furnace, close the tube furnace, and then fill with ammonia gas for 10 minutes to replace the air in the tube furnace to avoid air affecting the test results. Then, when the furnace temperature of the tubular constant temperature furnace gradually reaches three specific temperatures of 750°C, 800°C and 850°C, the ammonia gas valve is opened for ammoniation, the ammonia gas flow rate is 500ml / min, and the nitriding time is 2h. Cool naturally in the furnace. A block-shaped sample of vegetable pine is obtained, and the sample is finely ground to obtain a product powder. After quantitative analysis of the phase, the result is: the main phase is Mg 3 N 2 phase, followed by the MgO phase. as attached figure 1 shown.
[0024] From the results obtained in Example 1, the generated Mg 3 N 2 The particles are less uniform, prone to caking, and the purity is...
Embodiment 2
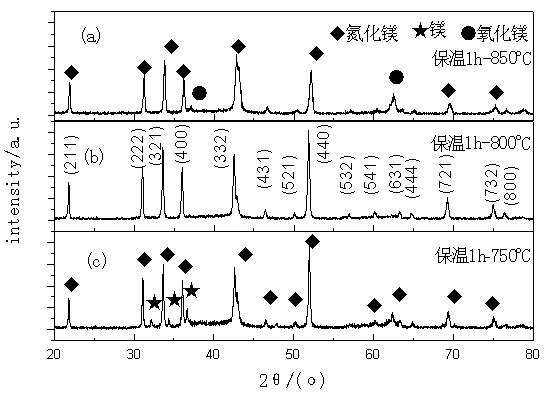
[0026] Put 1.5g of Mg powder in a ceramic boat, put the ceramic boat into the tube furnace, close the tube furnace, and then fill with ammonia gas for 10min to replace the air in the tube furnace to avoid air affecting the test results, and then When the furnace temperature of the tubular thermostatic furnace reaches 600°C, keep it in an ammonia atmosphere for 60 minutes, and then gradually increase the tubular thermostatic furnace to three specific temperatures of 750°C, 800°C, and 850°C, and open the ammonia valve for ammoniation. , ammonia flow 500ml / min, nitriding time 60min, after nitriding, the ceramic boat is naturally cooled in the furnace. A block-shaped sample of vegetable pine is obtained, and the sample is finely ground to obtain a product powder. Quantitative analysis of the phase shows that the pure phase is Mg when the sample is kept at 600°C for 1h and then nitrided at 800°C for 1h. 3 N 2 Mutually. as attached figure 2 (b) shown.
Embodiment 3
[0028] Put 2g of Mg powder in a ceramic boat, put the ceramic boat into the tube furnace, close the tube furnace, and then fill with ammonia gas for 15min to replace the air in the tube furnace to avoid air affecting the test results, and then wait for When the furnace temperature of the tubular constant temperature furnace reaches 610°C, keep it in an ammonia atmosphere for 40 minutes, and then gradually increase the tubular constant temperature furnace to three specific temperatures of 750°C, 800°C, and 850°C, and open the ammonia valve for ammoniation. The flow rate of ammonia gas was 400ml / min, and the nitriding time was 70min. After nitriding, the ceramic boat was naturally cooled in the furnace. A block-shaped sample of vegetable pine is obtained, and the sample is finely ground to obtain a product powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com