Solid-phase synthesis method of artificial E selectin
A solid-phase synthesis and selectin technology, which is applied in the preparation methods of peptides, chemical instruments and methods, animal/human proteins, etc., to achieve the effects of short production cycle, low production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1E-selectin (YTHLVIQ-NH 2 ) preparation
[0052] (1) Raw material preparation and identification:
[0053] Rink Amide-MBHA Resin: Product NO.: HCRAm04-1-1, Lot NO.: GRMH0706, specifications: 0.42mmol / g, 100-200mesh, 1% DVB, manufacturer: Hecheng (Tianj ing Nankai Hecheng Sci&Tech.Co .Ltd.).
[0054] Protected amino acid raw materials were purchased from Applied Biosystems and Jill Biochemical in the United States, and these raw materials needed to be identified. The identification results are shown in Table 1, and the purchased raw materials met the requirements.
[0055] Table 1 Determination results of protected amino acid raw materials
[0056]
[0057] DMF treatment: Soak in 3A molecular sieve, use FDNB (2,4 nitrofluorobenzene) OD≤0.15. (DMF used below is all processed DMF).
[0058] Hexahydropyridine treatment: redistilled.
[0059] (2) Concrete synthetic steps:
[0060] 1) Synthesis of Fmoc-Q Trt - Resin:
[0061] De-Fmoc step: Weigh 5g (0.4...
Embodiment 2
[0095] Embodiment 2E-selectin (YTHLVIQ-NH 2 ) preparation
[0096] Material preparation is similar to Example 1, E-selectin (YTHLVIQ-NH 2 ) was prepared on a multiplex polypeptide synthesizer. The peptide grafting reaction was carried out at 30°C for 2-3 hours. The final yield was 67.33%.
Embodiment 3
[0097] Embodiment 3E-selectin (YTHLVIQ-NH 2 ) purification
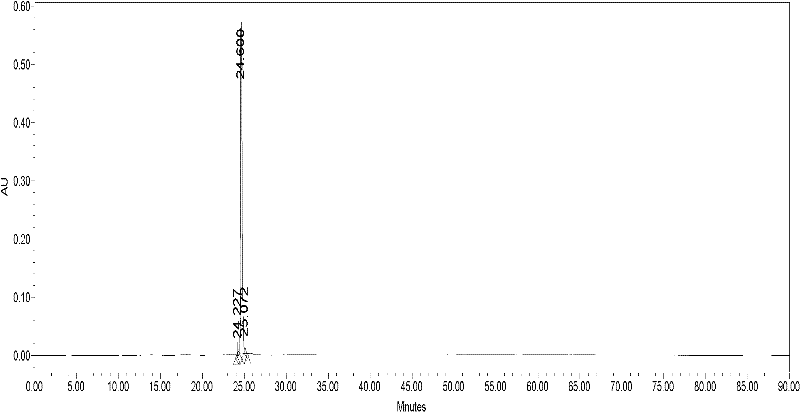
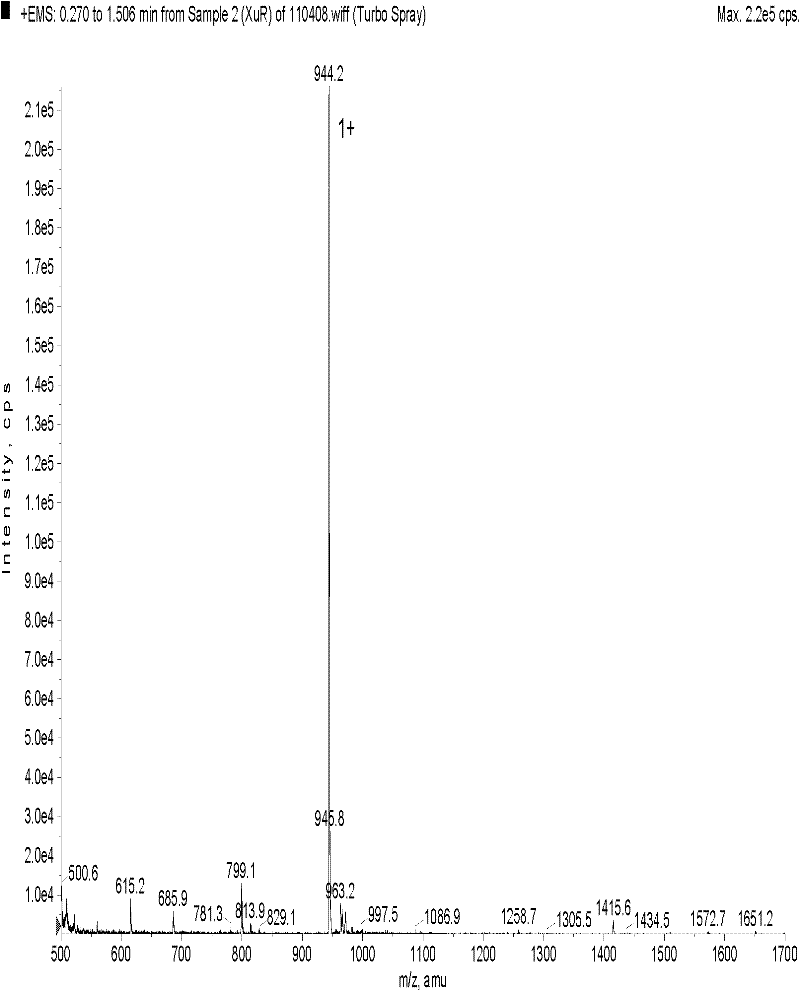
[0098] The prepared crude peptide was subjected to column chromatography with TSK 40S (D=2.5cm, H=85cm, V=240ml), 1N HAC containing 5% acetonitrile as eluent, flow rate 1.5ml / min, and the recovery rate was 50%. Conditions of HPLC purification: Loading amount: 100mg, dissolved in 50% acetic acid containing 5% acetonitrile; Buffer: 1N acetic acid containing 5% acetonitrile; flow rate 1.5ml / min; detection wavelength 280nm; there are three peaks P1, P2, P3 , collected P2, and freeze-dried to obtain a soft solid. Such as figure 1 , with a purity of 97%. TLC thin-plate chromatography conditions used for purification (DCM:MeOH:HAC=90:8:2). HPLC high performance liquid chromatography, C18 column, Buffer A 0.1% TFA, Buffer B is 100% acetonitrile containing 0.1% TFA, elution gradient: 0-5mins, 0% Buffer B; 5-35mins, 40%-100% Buffer B; 35-45mins, 100% Buffer B; 45-50mins, 100% Buffer A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com