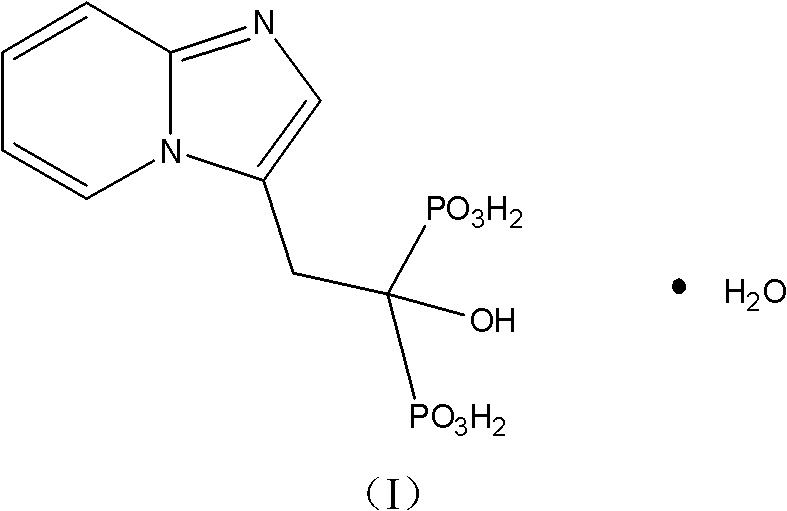
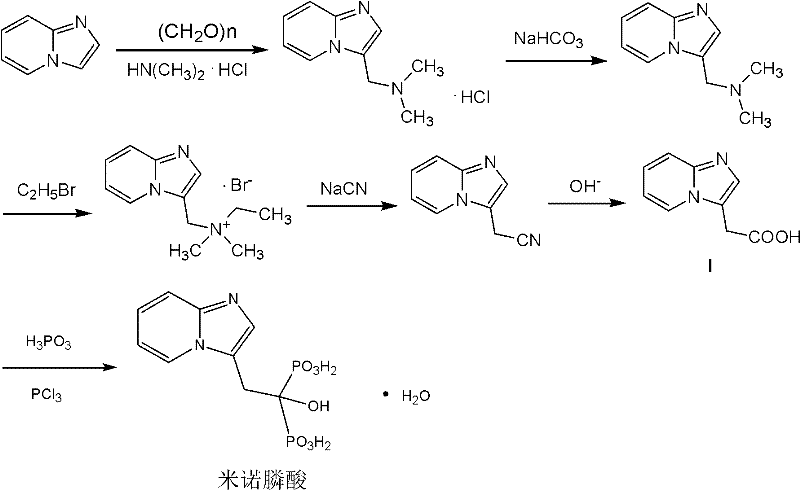
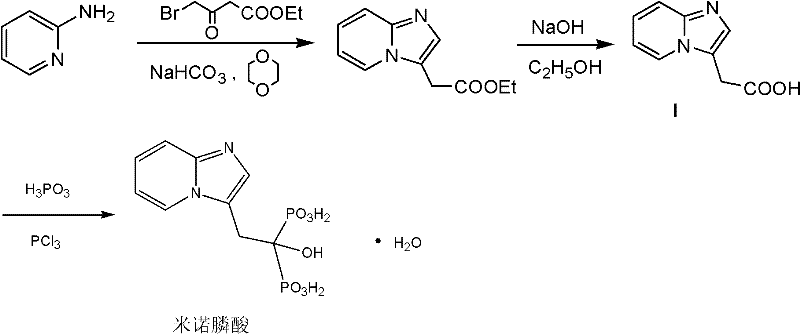
Method for preparing minodronic acid intermediate
A technology of hydrochloric acid and acetic acid, applied in directions such as organic chemistry, can solve the problems of low bromination yield, unsuitable for industrial production, large amount of solvent, etc., and achieves the effects of excellent product quality, cheap raw materials, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of Diethyl 2-(imidazo[1,2-a]pyridin-3-yl)malonate(III)
[0033] Dissolve 50ml of diethyl malonate in 1L of absolute ethanol, add 30g of sodium ethoxide and stir at room temperature for 2h, add 50g of 3-bromo-imidazo[1,2-a]pyridine (II), and reflux for 4h. The reaction solution was poured into a large amount of ice water, extracted with ethyl acetate, the organic layers were combined, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and air-dried at 60°C to obtain 58 g of a yellow solid with a yield of 82.8%. Preparation of 2-(imidazo[1,2-a]pyridin-3-yl)acetic acid (I)
[0034] Add 58g of intermediate (III) into the reaction flask, add 348ml of concentrated hydrochloric acid, raise the temperature to 120°C and stir for 6h to complete the reaction. The reaction solution was concentrated under reduced pressure to obtain 27.5 g of khaki solid (I), with a yield of 75.3%.
Embodiment 2
[0036] Recrystallization of 2-(imidazo[1,2-a]pyridin-3-yl)acetic acid (I)
[0037] Add 27.5g of intermediate I crude product to the reaction flask, add 550ml of methanol, heat to dissolve, filter while hot, cool and crystallize the filtrate, and filter with suction to obtain 23.8g of off-white solid with a yield of 86.5% and a melting point of 236°C-238°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com