Method for expressing Reteplase in Escherichia coli by using dual plasmid system
A technology of Escherichia coli and recombinant Escherichia coli, applied in the field of biomedicine, can solve the problems of lack, verification of thrombolytic activity, inability to ensure clinical application, etc., and achieve the effects of low cost, simple production process, and efficient soluble expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Construction of rPA genetically engineered bacteria
[0032] 1. Using the pMD18T-tPA plasmid constructed in the previous research of our laboratory as a template (Zhen Jie, Jiang Chengying, Du Lianxiang, etc.. Reteplase gene cloning and expression in Pichia methanolica. Journal of South China University of Technology Natural Science Edition, 2006, 34(12): 25-29).
[0033] Using upstream primers:
[0034] 5’-CGC GGATCC ATCTTACCAAGGAAACAGTGACTGCTAC-3’ (NO3);
[0035] Downstream primer:
[0036] 5’-GCC AAGCTT CAATGTCTTACCAAGGAAACAGTGACTGC-3’ (NO4);
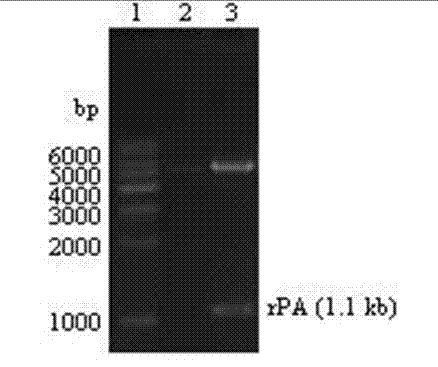
[0037] Carry out PCR amplification, the amplified PCR products are detected by agarose gel electrophoresis, and then purified and recovered by gel purification to obtain rPA gene fragments.
[0038] In order to facilitate subsequent operations, the upstream and downstream primers were introduced Bam HⅠand Hin dⅢ restriction site (underlined part).
[0039] The sequence comparison confirmed that the rPA coding gene sequence obtained by ...
Embodiment 2
[0046] Induced expression of rPA in E. coli
[0047] 1. Inoculate the recombinant E. coli co-transformed with pET22b-rPA and pET40b plasmids into 5 mL of LB liquid medium containing a final concentration of 50 μg / mL Amp and 30 μg / mL Kan, and cultivate overnight at 37°C and 220 rpm.
[0048] 2. Transfer the overnight cultures obtained in the previous step to 200 mL of LB liquid medium at a volume ratio of 1%, and then add IPTG (final concentration 0.6 mM) to induce induction at 25°C when the OD600 is 0.6 Express for 3 h.
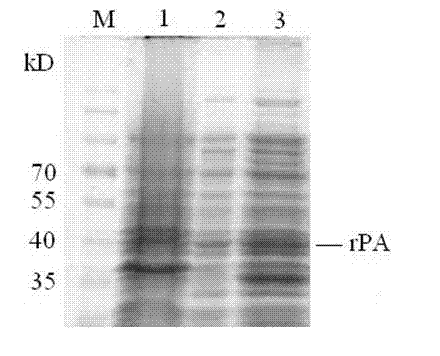
[0049] 3. After the induction of expression, take 3 mL of bacterial solution and centrifuge at 12000 rpm to collect the bacterial cells, add SDS loading buffer, and boil to collect total protein. The remaining bacterial liquid was collected by centrifugation at 12000 rpm and broken by ultrasonic method. After centrifugation at 12000 rpm, the supernatant (soluble protein) and precipitate (protein in the form of inclusion bodies) were detected by SDS-PAGE...
Embodiment 3
[0052] Recombinant protein thrombolytic activity detection
[0053] 1. Preparation of fibrin plate: Weigh 0.02 g of fibrinogen and add it to a small conical flask containing 5 mL of PBS, mix well and place it at 37°C for 10 min to preheat it to dissolve it. At the same time, take another appropriate amount of thrombin into an EP tube containing 1mL PBS, blow gently, and put it in 37℃ to preheat.
[0054] 2. Weigh out 0.07 g of agarose and dissolve it with 7 mL PBS, heat to dissolve, and then cool to an appropriate temperature. The thrombin solution was quickly mixed into the fibrinogen solution, mixed immediately, and then added to the agarose solution. After shaking, the mixture was poured into a plate to cool and solidify.
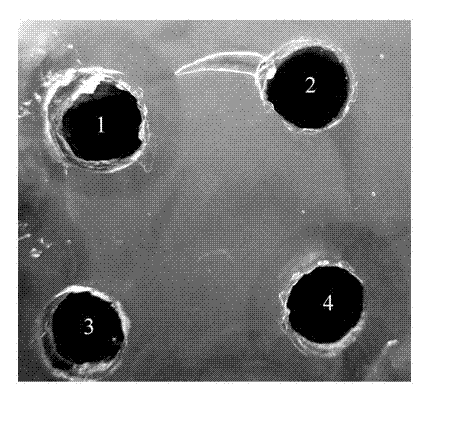
[0055] 3. Use a puncher to punch holes in the prepared fibrin plate, add 30 μL of the sample to be tested, and incubate at 37°C for 12 h to detect the size of the thrombolytic circle. In the experiment, the induced expression products of pET22b and pET40b tra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com