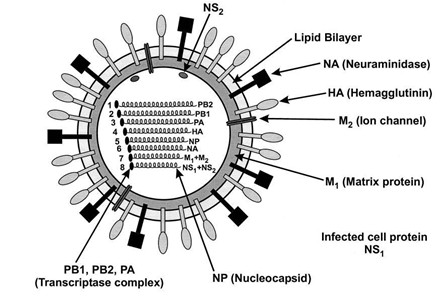
Nucleic acid dual fluorescence PCR (Polymerase Chain Reaction) detection kit for influenza A/B virus
A type of influenza B virus and detection kit technology, applied in the biological field, can solve the problems of time-consuming, complicated and expensive gene sequence determination methods, reduce the chance of pollution, and achieve fast, objective and repeatable detection results Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Influenza A / B nucleic acid detection kit
[0040] The double fluorescent quantitative PCR detection kit of influenza A / B nucleic acid of the present embodiment comprises RNase inhibitor, RT-PCR reaction solution, enzyme mixture, double reaction solution of influenza A / B virus, positive control and negative control,
[0041] Wherein, the RNase inhibitor is DEPC water; the RT-PCR reaction solution includes 10× buffer, MgCl2 and dNTPs;
[0042] The Influenza A / B Duplex Reaction Solution includes the following components:
[0043]Component (1): composed of a pair of primers for detecting influenza A virus and a probe for detecting influenza A virus; wherein, the base sequences of the two primers are SEQ ID No.1 and SEQ ID No.2 respectively shown; the base sequence of the probe is shown in SEQ ID No.3, the 5' end of the probe is marked with a fluorescent reporter group, and the 3' end is marked with a fluorescent quencher group; the primer for detecting influenza...
Embodiment 2
[0063] Embodiment 2 Sensitivity test
[0064] The positive reference product is the inactivated virus culture solution, which comes from the Jiangsu Provincial Center for Disease Control and Prevention.
[0065] The negative reference product is RNase-free water. Weigh 1g of DEPC with an electronic balance, add purified water to 1000ml and mix well, then sterilize at 121°C / 20 minutes in a sterilizing pot, mark it, and store it at room temperature.
[0066] The kit of the present invention is used for detection.
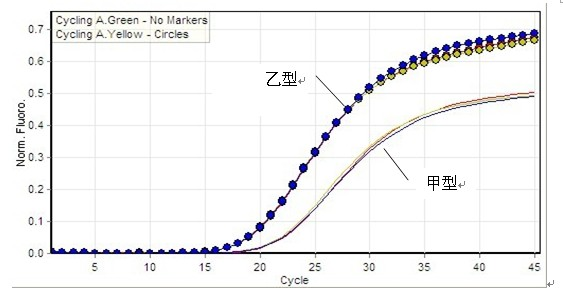
[0067] The test results show that the kit of the present invention has good sensitivity, and the CT value changes in a gradient as the concentration decreases ( Figure 4 , Figure 5 ). The test result shows that the kit of the present invention has high sensitivity for the diagnosis of type A / type B influenza virus.
[0068]
Embodiment 3
[0069] Embodiment 3 specificity test
[0070] In order to detect the specificity of the type A / B influenza virus detection kit of the present invention, the type A / B influenza virus detection kit of the present invention is used to detect respiratory syncytial virus, human adenovirus, and human parainfluenza virus.
[0071] The test results show that: the FAM channel only amplifies influenza A virus ( Figure 6 ), the HEX channel only amplifies influenza B virus ( Figure 7 ). It shows that the detection kit of the present invention can specifically amplify influenza virus without cross-reaction with other viral nucleic acids.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com