Lithium-rich cathode material of lithium ion battery and preparation method thereof
A lithium-rich cathode material, lithium-ion battery technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of large solvent consumption, difficult to strictly control the proportion of transition metal salts, poor repeatability of precursors, etc., to achieve the preparation method Simple and easy to implement, suitable for large-scale production, and the effect of excellent electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, preparation Li[Li 0.2 Ni 0.13 mn 0.54 co 0.13 ]O 2
[0029] Press LiNO 3 , Ni(NO 3 ) 2 , Mn(NO 3 ) 2 , Co(NO 3 ) 2 、C 6 h 8 o 7 The molar ratio is 1.25: 0.13: 0.54: 0.13: 1.2, take water as solvent, mix well, adjust the pH to 7; react at 50°C for 10 hours to obtain a transparent sol; dry the sol at 80°C for 120 Hours, the precursor powder was obtained; the precursor powder was pre-sintered at 300°C for 8h to obtain the intermediate product powder, and the intermediate product was pressed into tablets and then sintered at 900°C for 12h to obtain the product Li[Li 0.2 Ni 0.13 mn 0.54 co 0.13 ]O 2 .
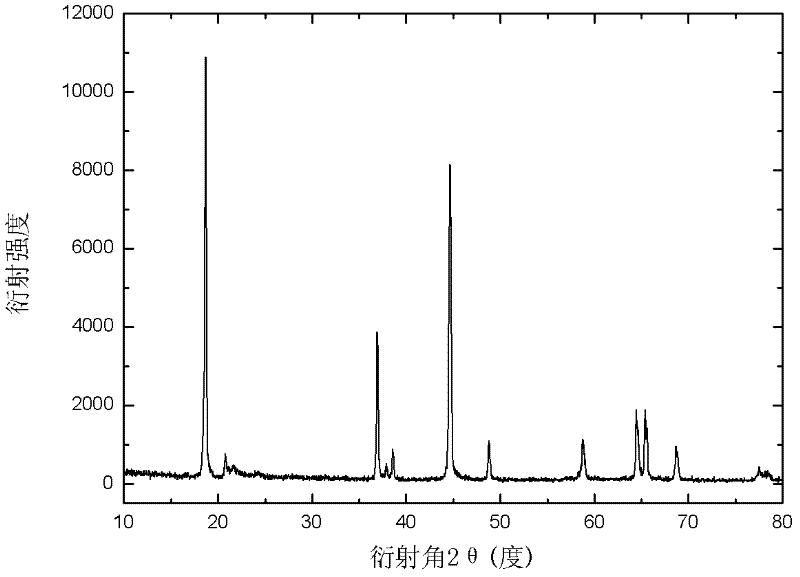
[0030] Powder X-ray diffractometer (Rigaku DmaxrB, CuK α X-ray) analysis confirms the structure, the results are as follows figure 1 shown, from figure 1 It can be seen that there is no impurity peak in the spectrogram, indicating that the product is of high purity.
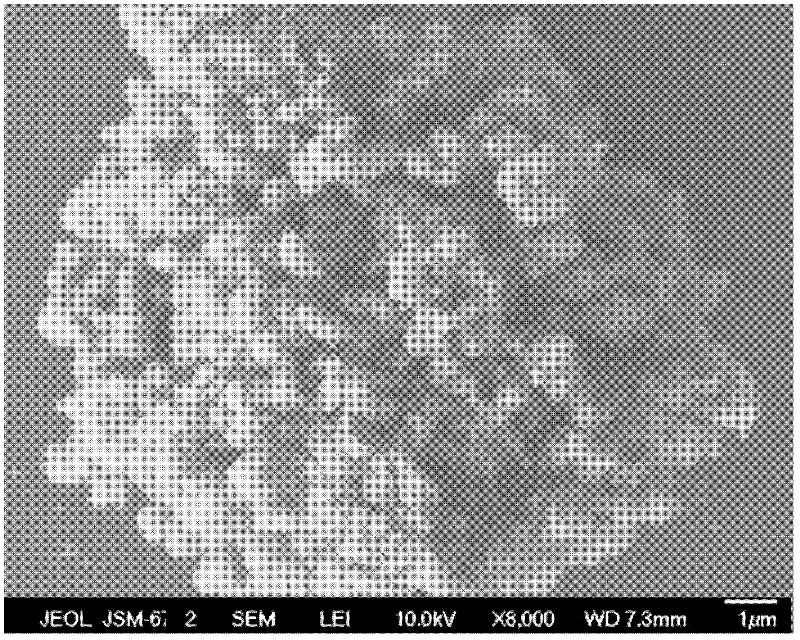
[0031] The morphology of the lithium-rich cathode material was character...
Embodiment 2
[0033] Embodiment 2, preparation Li[Li 0.25 Ni 0.04 mn 0.54 co 0.17 ]O 2
[0034] Press LiNO 3 : Ni(NO 3 ) 2 : Mn(NO 3 ) 2 : Co(NO 3 ) 2 : C 6 h 8 o 7 The molar ratio is 1.30:0.04:0.54:0.17:1, and the solution obtained by water and ethanol according to the volume ratio of 1:1 is used as a solvent, mixed uniformly, and the pH is adjusted to 6; reacted at 50°C for 10 hours to obtain Transparent sol; dry the sol at 80°C for 120 hours to obtain a precursor powder; pre-sinter the precursor powder at 400°C for 6 hours to obtain an intermediate product powder; press the intermediate product powder and sinter at 900°C 10h promptly obtains product Li [Li 0.25 Ni 0.04 mn 0.54 co 0.17 ]O 2 Nanoparticles, the particle size is 20nm-1000nm.
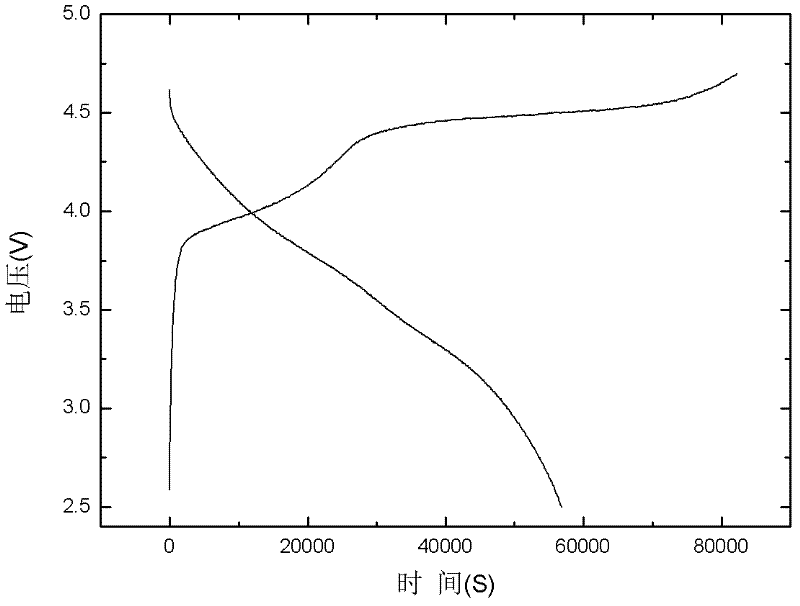
[0035] The specific discharge capacity of the lithium-rich cathode material prepared in this example is 215mAh / g.
Embodiment 3
[0036] Embodiment 3, preparation Li[Li 0.2 Ni 0.06 mn 0.47 co 0.27 ]O 2
[0037] Press LiNO 3 : Ni(NO 3 ) 2 : Mn(NO 3 ) 2 : Co(NO 3 ) 2 : C 6 h 8 o 7 The molar ratio is 1.23: 0.06: 0.47: 0.27: 0.8, and the solution obtained by mixing water and ethylene glycol according to the volume ratio of 3: 1 is used as a solvent, mix well, adjust the pH to 5; react at 60 ° C for 8 hours , to obtain a transparent sol; dry the sol at 80°C for 120 hours to obtain a precursor powder; pre-sinter the precursor powder at 500°C for 5 hours to obtain an intermediate product powder; Sintered for 18h to obtain the product Li[Li 0.2 Ni 0.06 mn 0.47 co 0.27 ]O 2 Nanoparticles, the particle size is 20nm-1200nm.
[0038] The discharge specific capacity of the lithium-rich cathode material prepared in this example is 239mAh / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com