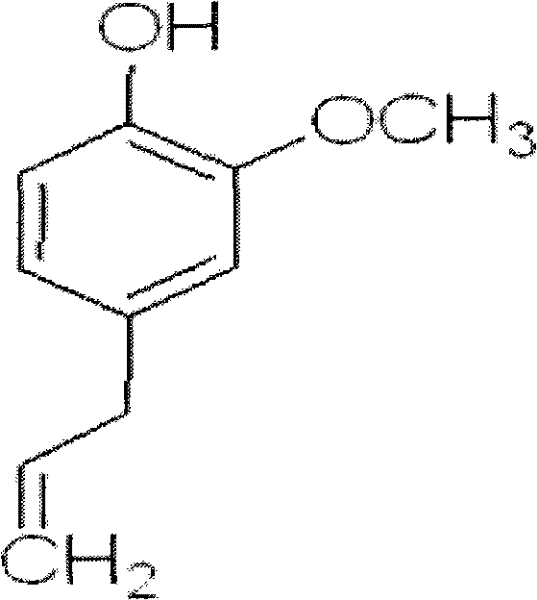
Use of clove oil, eugenol and derivatives thereof in the preparation of medicines for histamine H3 receptor antagonists or inverse agonists
A technology of inverse agonists and receptor antagonists, applied in the direction of drug combinations, pharmaceutical formulas, plant raw materials, etc., can solve the problems of poor penetration of the central nervous system and unfavorable treatment of central nervous system diseases, and achieve clear drug effects, Quality Controlled Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 clove oil, eugenol and eugenol derivative
[0028] Clove oil, eugenol and eugenol derivatives are commercially available and can be purchased directly.
[0029]The preparation method of clove oil: After pulverizing cloves, weigh 500g and place it in a 2000ml flask, add 3 times of water and soak for 60min, then add 15% NaCl solution as an auxiliary agent, and heat to keep the system in a boiling state. The clove oil-water emulsion that evaporates is moved in the centrifuge, 4000r / min centrifugal separation. Drain the water in the upper layer to obtain the volume of clove oil, and the yield of clove volatile oil is 2.26%. Through HPLC detection, the eugenol content is greater than 80%.
[0030] Except adopting this extraction method to prepare, also can adopt supercritical fluid (CO2) extraction method (Yuan Yujie etc., the supercritical CO2 extraction process research of clove oil and GC-MS analysis, Chinese natural medicine, the 5th peri...
Embodiment 2
[0032] The histamine H3 receptor in vitro affinity determination of embodiment 2 clove oil, eugenol and eugenol derivative
[0033] 1. Experimental method
[0034] (1) Take the frontal brain of the rat under freezing, and put it into 20 times the solution containing 2mM MgCl 2 and 50mM TrisHCl, the pH was adjusted to 7.4, and the homogenate was centrifuged at 45,000G for 10 minutes. Remove the supernatant, pass through a Polytron, and resuspend the cell membrane pellet in a solution containing 2 mM MgCl 2 and 50mM Tris HCl, the pH was adjusted to 7.4, and centrifuged again. At a concentration of 12 mg / ml, the final pellet was resuspended in a solution containing 2 mM MgCl 2 and 50mM Tris HCl, the pH was adjusted to 7.4, and the temperature was adjusted to 25°C;
[0035] (2) Dilutions of clove oil, eugenol and eugenol derivatives were prepared in 10% DMSO / 50mM Tris (pH 7.4) to prepare serial concentration test solutions respectively. Put 25 microliters of the test solution...
Embodiment 3
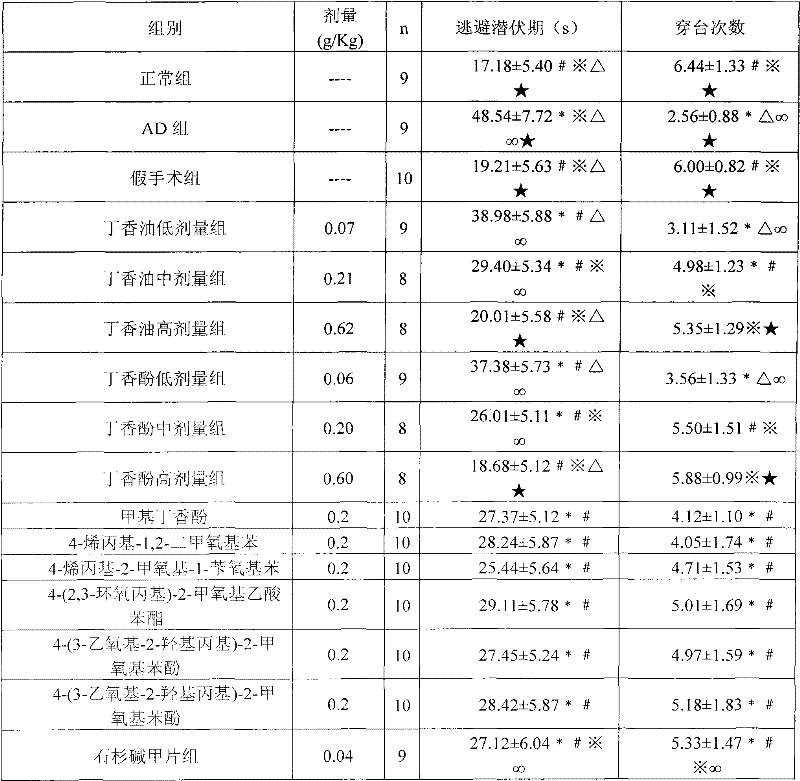
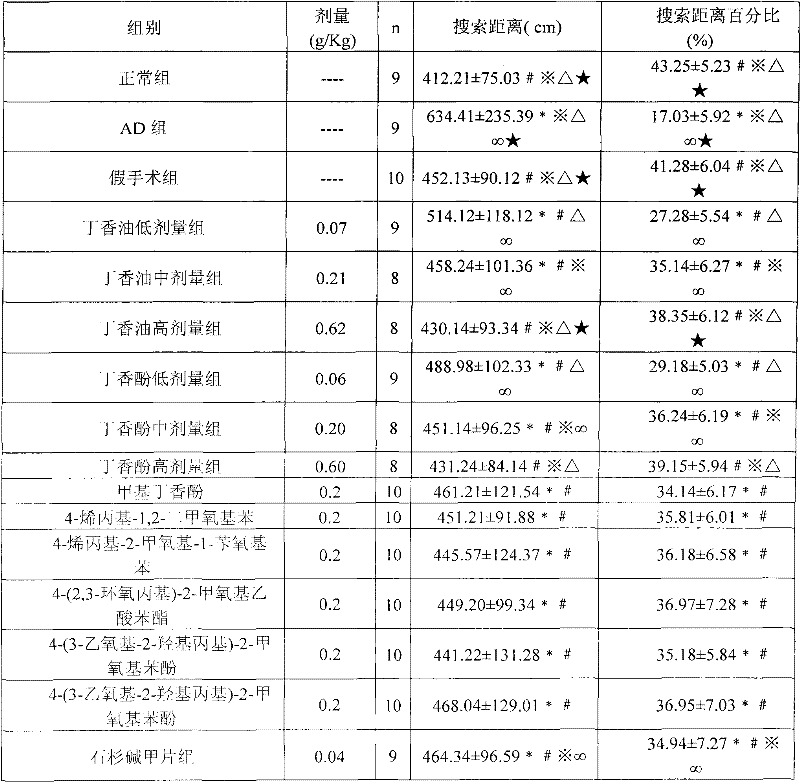
[0043] Example 3 Effect of Clove Oil, Eugenol and Eugenol Derivatives on Alzheimer's Disease
[0044] 1. Grouping of animals: Wistar rats, half male and half female, weighing (220±20) g, were purchased from the Animal Center of Sichuan University. After 1 week of adaptive feeding, they were randomly divided into: normal group; AD group (both dorsal hippocampus injected with Aβ 25-35 ); sham operation group (1 μl normal saline was injected into bilateral dorsal hippocampus); low-dose clove oil group, middle-dose clove oil group, high-dose clove oil group, low-dose eugenol group, middle-dose eugenol group, and high-dose eugenol group dosage group, methyl eugenol group, 4-allyl-1,2-dimethoxybenzene group, 4-allyl-2-methoxyl-1-benzyloxybenzene group, 4-(2 , 3-epoxypropyl)-2-methoxyphenyl acetate group, 4-(3-ethoxy-2-hydroxypropyl)-2-methoxyphenol group, 4-(3-ethoxy Base-2-hydroxypropyl)-2-methoxyphenol group and huperzine A group, bilateral dorsal hippocampus injected with 1μl s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com