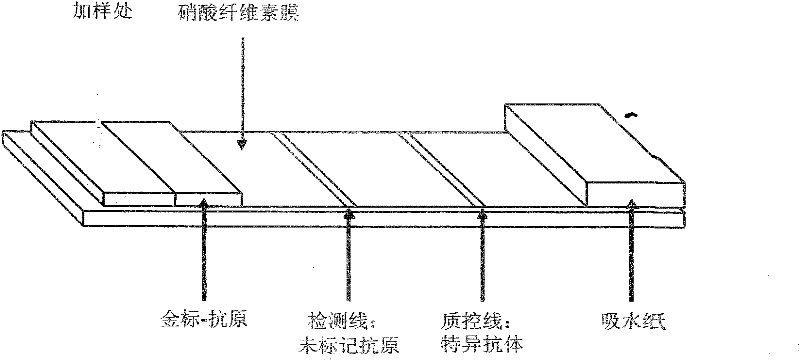
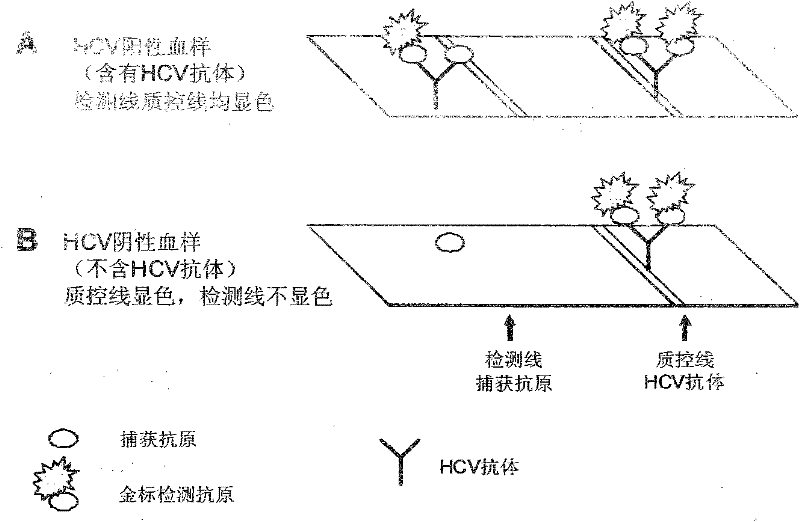
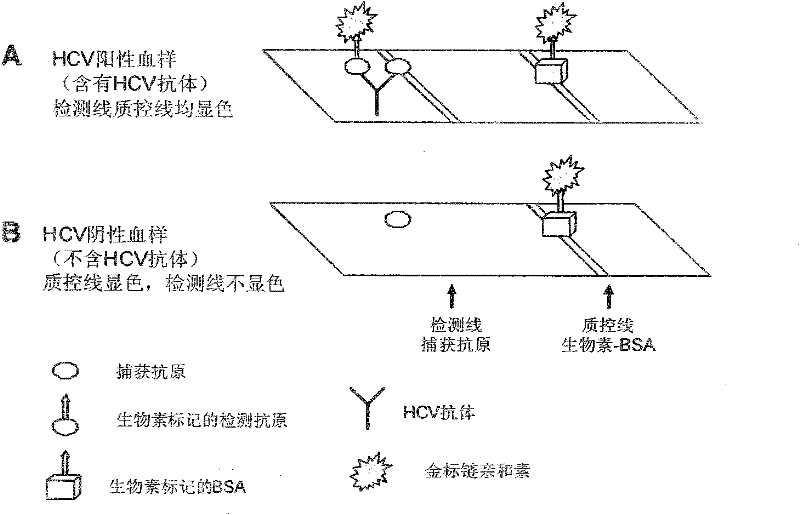
Direct Application of Recombinant Fusion Proteins in Rapid Diagnostic Tests
A technology of fusion protein and protein, applied in the field of bioengineering, can solve problems such as water solubility decline, and achieve the effect of convenient purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Directly using the purified His-MBP-HIV fusion protein for rapid detection of HIV planar flow type
[0036] (1) Construction of His-HIV and His-MBP-HIV fusion genes
[0037] Different cDNA fragments of HIV virus (gp41 of HIV-1 and gp36 of HIV-2) were amplified by PCR and cloned into Escherichia coli expression vectors pET-28 and pET-32. Such as Figure 4 As shown, HIV recombinant genes A and B contain the same HIV viral gene sequence, and recombinant genes C and D contain the same HIV viral gene sequence, but the N-terminals of A and C only carry His-tag, while the N-terminals of B and D Carry His-tag and MBP fusion gene.
[0038] (2) His-MBP-HIV recombinant fusion protein is improved in water solubility and purity than His-HIV recombinant protein
[0039] The recombinant expression plasmids containing the above-mentioned A, B, C and D were transformed into Escherichia coli strain BL-21, and the expression was induced by IPTG to produce the recombinant pro...
Embodiment 2
[0047]Example 2: Directly using the purified His-MBP-HCV fusion protein for rapid detection of HCV planar flow type
[0048] (1) Construction of His-HCV and His-MBP-HCV fusion genes
[0049] Different HCV viral cDNA fragments (HCV genotypes I and II; gene fragments encoding core antigen, NS3, NS4 and NS5 nonstructural proteins) were amplified by PCR and cloned into E. coli expression vectors pET-28 and pET-32. Such as Figure 6 As shown, HCV recombinant genes A and B contain the same HCV viral gene sequence, and recombinant genes C and D contain the same HCV viral gene sequence, but the N-terminals of A and C only carry His-tag, while the N-terminals of B and D Carry His-tag and MBP fusion gene.
[0050] (2) His-MBP-HCV recombinant fusion protein is improved in water solubility and purity than His-HCV recombinant protein
[0051] The above-mentioned expression plasmids encoding HCV recombinant proteins A, B, C and D were transformed into Escherichia coli strain BL-21, and t...
Embodiment 3
[0059] Example 3: Using the purified His-MBP-HIV and His-MBP-HCV recombinant fusion protein for double rapid detection of planar flow type HIV / HCV
[0060] (1) Preparation of biotin-labeled His-MBP-HIV and His-MBP-HCV recombinant fusion protein and BSA
[0061] The HIV recombinant fusion protein B (His-MBP-gp36-gp41) in Example 1 was labeled with Sulfo-LC-NHS-biotin using the method described in Example 1 (3) and Example 2 (3) respectively and implemented The HCV recombinant fusion protein D (His-MBP-NS5-NS4-NS3-C8 asp) in Example 2 was used as the detection antigen. In the same principle, BSA was labeled with Sulfo-LC-NHS-biotin to make a quality control line.
[0062] (2) Preparation of gold-labeled streptavidin: colloidal gold suspension was prepared according to the method described in Example 1 (4), and colloidal gold was used to label streptavidin.
[0063] (3) Prepare detection test strips with His-MBP-HIV and His-MBP-HCV recombinant fusion protein
[0064] Use the H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com