A method for preparing oil-soluble iron ferric oxide nanoparticles by oil-water interface method
A technology of ferric oxide, oil-water interface method, applied in the direction of iron oxide/iron hydroxide, ferrous oxide, etc., can solve the problems of product purity not easy to be controlled, troublesome synthesis steps, poor particle dispersibility, etc. Achieve the effect of good particle uniformity and dispersion, easy operation and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
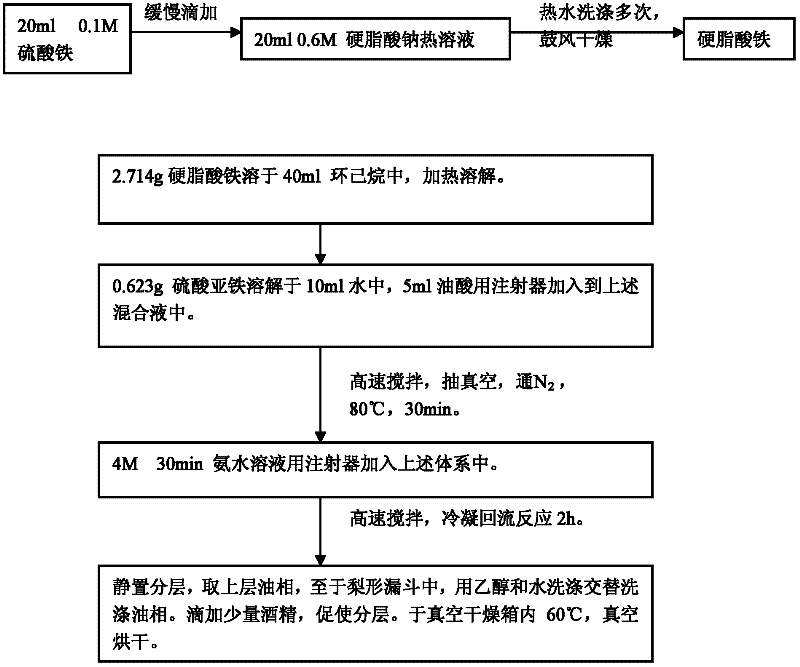
[0028] 1) Weigh 0.7g of ferric sulfate and dissolve it in 30ml of deionized water; Weigh 906.9g of sodium stearate and dissolve it into 60ml of aqueous solution under magnetic stirring and heating. The ferric sulfate solution was added dropwise to sodium stearate and reacted at 60°C for 30 minutes. After the reaction is over, the reactant is taken, filtered with a Buchner funnel, and washed 3 times with hot water. Put it in a blast drying box and dry for 24 hours.
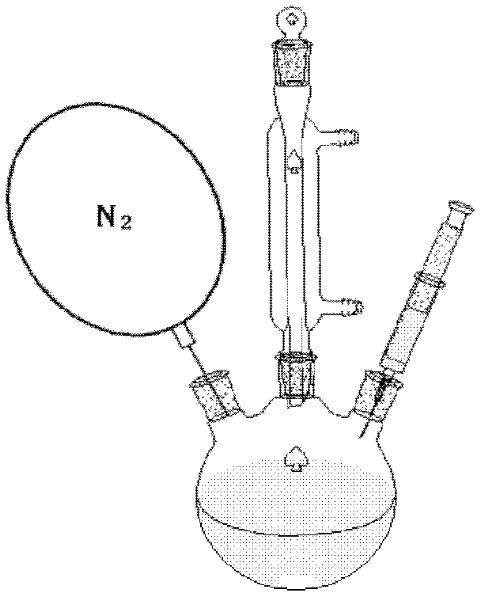
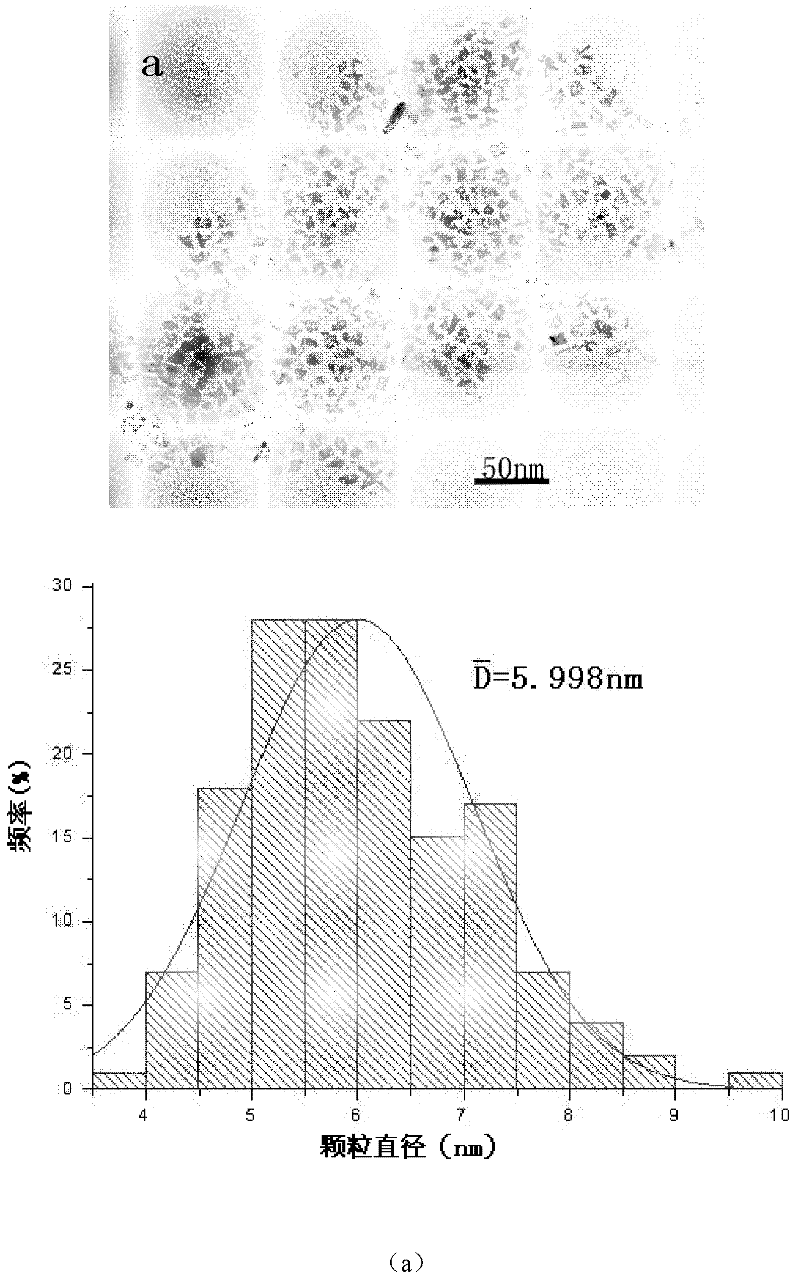
[0029] (2) Weigh 2.715g of the above iron stearate solution, 0.003mol, and dissolve it in 40ml of cyclohexane. After heating to dissolve, a 0.075mol / L iron stearate solution is prepared. Then use a syringe to inject 5ml of oleic acid into a three-necked flask. Vacuum is maintained at -0.9Pa for 2 minutes. High-purity nitrogen gas was blown into it and maintained for 20 minutes under high-speed magnetic stirring (v=400rpm). Connect the reflux tube, keep the reactor in an oxygen-free state and pure nitrogen state, a...
Embodiment 2
[0034] (1) Weigh 0.35 g of ferric sulfate and dissolve in 30 ml of deionized water; weigh and dissolve 453.5 g of sodium stearate into 60 ml of aqueous solution under magnetic stirring and heating. The ferric sulfate solution was added dropwise to sodium stearate and reacted at 80°C for 40 minutes. After the reaction is over, the reactant is taken, filtered with a Buchner funnel, and washed 3 times with hot water. Put it in a blast drying box and dry for 24 hours.
[0035] (2) Weigh 1.375g of the above-mentioned iron stearate solution and dissolve it in 40ml of cyclohexane. After heating and dissolving, a 0.00375mol / L iron stearate solution is prepared. Then use a syringe to inject 2.5ml of oleic acid into a three-necked flask. Vacuum is maintained at -0.9Pa for 2 minutes. High-purity nitrogen gas was blown into it and maintained for 20 minutes under high-speed magnetic stirring (v=400rpm). Connect the reflux tube, keep the reactor in an oxygen-free state and pure nitrogen st...
Embodiment 3
[0040] (1) Weigh 0.7 g of ferric nitrate and dissolve in 30 ml of deionized water; weigh 1813.8 g of sodium stearate and dissolve it into 60 ml of aqueous solution under magnetic stirring and heating. The ferric sulfate solution was added dropwise to sodium stearate and reacted at 80°C for 40 minutes. After the reaction is over, the reactant is taken, filtered with a Buchner funnel, and washed 3 times with hot water. Put it in a blast drying box and dry for 24 hours.
[0041] (2) Weigh 2.715g of the above-mentioned iron stearate, 0.0015mol, and dissolve it into 40ml of cyclohexane. After heating and dissolving, a 0.00375mol / L iron stearate solution is prepared. Vacuum is maintained at -0.9Pa for 2 minutes. High-purity nitrogen gas was blown into it and maintained for 20 minutes under high-speed magnetic stirring (v=400rpm). Connect the reflux tube, keep the reactor in an oxygen-free state and pure nitrogen state, and stop stirring for 20 minutes to repel the gas in the reflux ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com