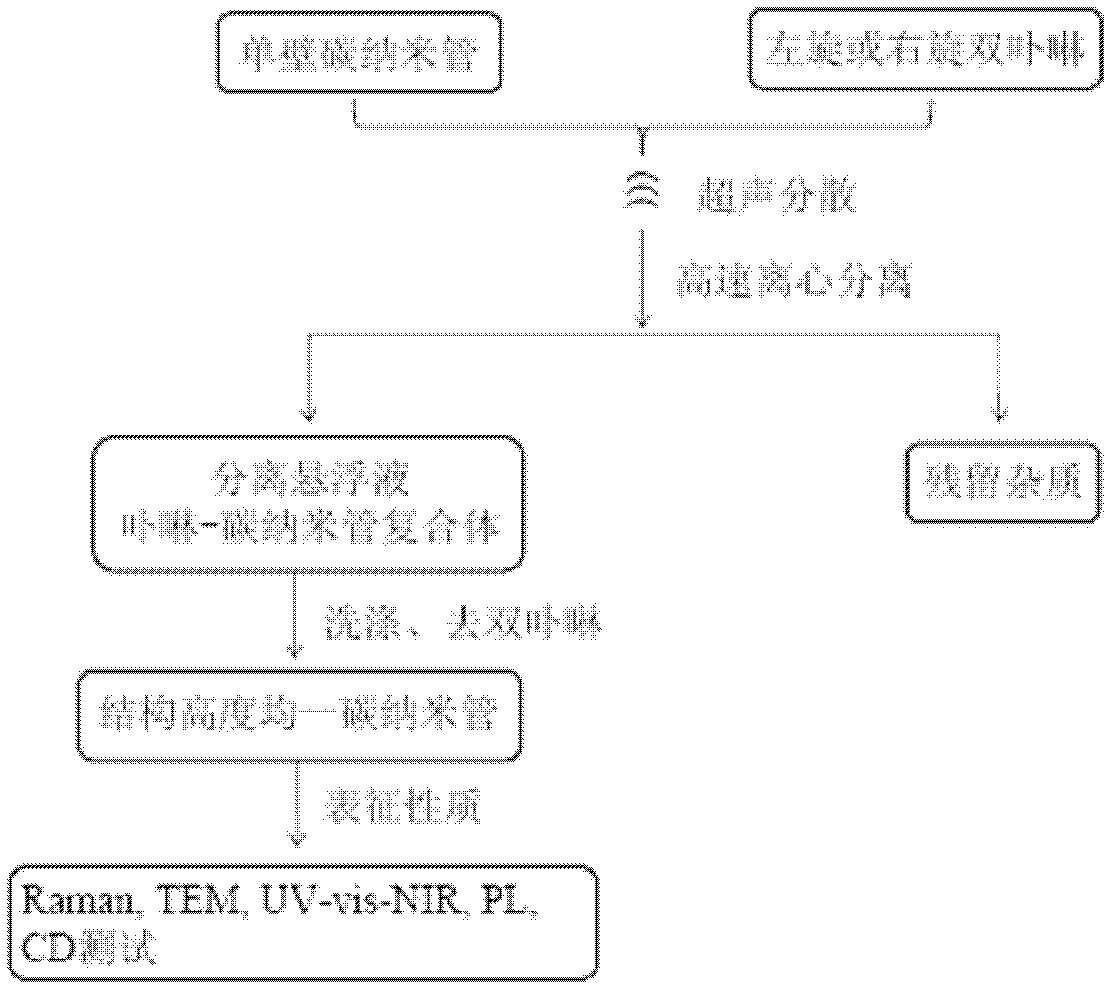
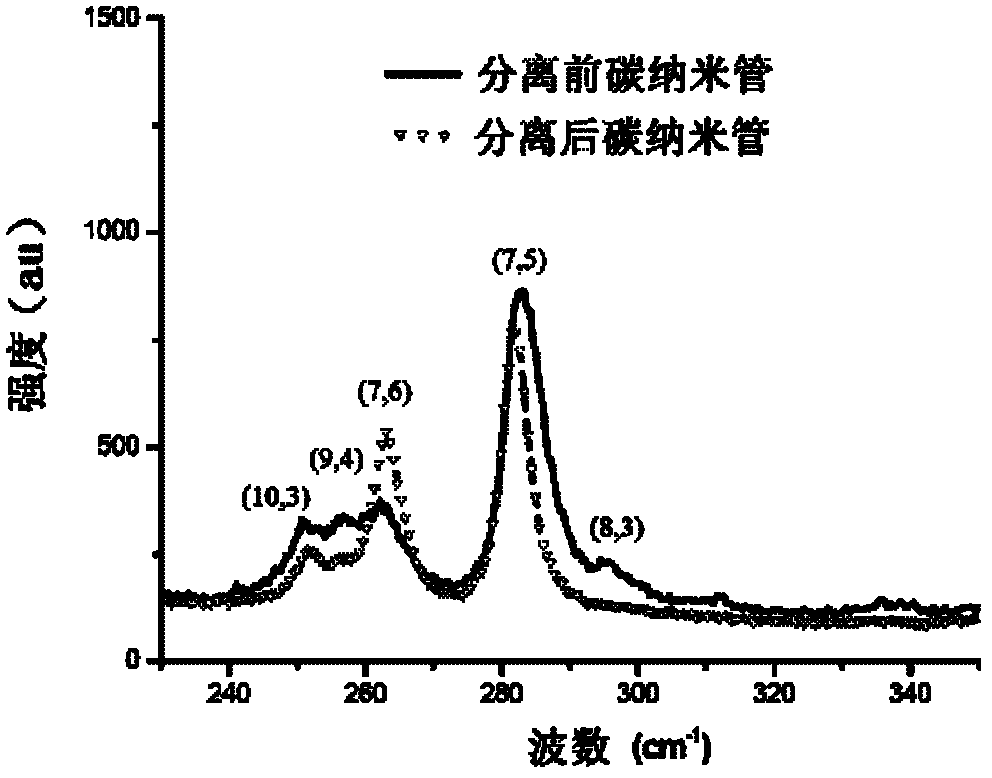
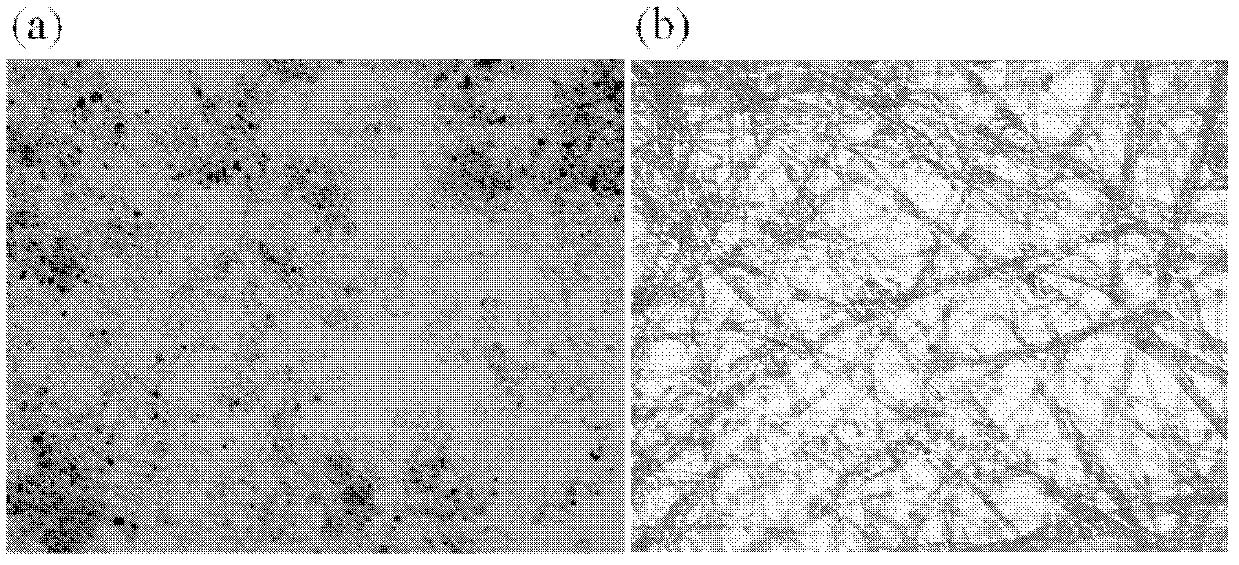
Chiral porphyrin complex nanotweezers and its preparation method and application in the separation of single-walled carbon nanotubes
A technology of carbon nanotubes and complexes, applied in the field of chiral porphyrin complex nanotweezers and its preparation, can solve the problems of complicated experimental steps, high separation cost, unfavorable separation of pure carbon nanotubes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 3,6-dibromo-N-substituted carbazole
[0031] According to the method disclosed in Macromolecules, 2002, 35, 6080, the preparation of 3,6-dibromo-N-n-octylcarbazole is taken as an example to illustrate.
[0032]
[0033] Add 60% sodium hydride (1.104 g, 27.6 mmol) and 25 ml of anhydrous tetrahydrofuran into a 200 ml three-necked flask, and start to add dropwise 3,6-dibromocarbazole (5 g, 15.4 mg) dissolved in 30 ml of tetrahydrofuran mol), about 30 minutes dropwise. After dropping, the cooling device was removed, and the temperature was gradually raised to reflux temperature, 4.5 ml of 1-bromo-n-octane (4.16 g, 25.2 mmol) was added, and the reaction was refluxed overnight. Pour the reaction solution into clear water, filter, wash until neutral, dry over anhydrous magnesium sulfate, remove the solvent, and use ethyl acetate and n-hexane mixed solvent (1:10) as eluent for further purification by column chromatography to obtain white Crystals, 80% yield....
Embodiment 2
[0036] Preparation of 3,6-dibromo-9,9-disubstituted fluorene
[0037] According to the method disclosed in Adv. Mater., 2008, 20, 2359, the preparation of 3,6-dibromo-9,9-di-2-ethylhexylfluorene is taken as an example to illustrate.
[0038]
[0039] Under argon protection, 60% sodium hydride (2.1 g, 52.5 mmol) and 50 ml of tetrahydrofuran were added to a 100 ml flask, and 3,6-dibromofluorene (4 g, 12.3 mmol) solution was stirred at room temperature for 2 hours. 5.4 g (28 mmol) of 1-bromo-2-ethylhexylane was added dropwise within 1 hour, and after the addition was complete, it was heated to reflux for 24 hours. Cool, neutralize with dilute hydrochloric acid, then extract with dichloromethane (3×50), combine the organic phases, dry over anhydrous magnesium sulfate, concentrate, and then separate by column chromatography (silica gel, petroleum ether) to obtain 6.50 g of free Color oil, yield 96.0%. Proton NMR spectrum analysis results: 1 HNMR (CDCl3, 270MHz), 7.80 (d, 2H), ...
Embodiment 3
[0042] Preparation of 2,8-dibromodibenzothiophene
[0043]
[0044] Prepare according to the method disclosed in J.Mater.Chem., 2003, 13, 1351, add dibenzothiophene (5 g, 27.1 mmol) in a 100 ml three-necked flask, 50 ml of carbon trichloride, cool to 0 ° C, The dropwise addition of liquid bromine (3.1 mL, 60.5 mmol) dissolved in 30 mL of carbon trichloride was started and continued for about 30 minutes. After dropping, the cooling device was removed, and the temperature was gradually raised to room temperature, and the reaction was carried out overnight. Pour the reaction solution into clean water, filter, wash until neutral, and dry. Recrystallized with absolute ethanol and dried to obtain white crystals with a yield of 80%. Proton NMR spectrum analysis results: 1 HNMR (CDCl3, 270MHz), 8.24 (m, 2H), 7.71 (m, 2H), 7.58 (m, 2H), nuclear magnetic resonance test shows that it is the target product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com