A kind of preparation method of the intermediate of rasagiline
A technology for intermediates and compounds, applied in the field of preparation of intermediates, can solve the problems of producing by-product boric acid, ineffective utilization of by-products, environmental pollution, etc., and achieves the effects of low production cost, reduction of the elimination of three industrial wastes, and environmental friendliness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
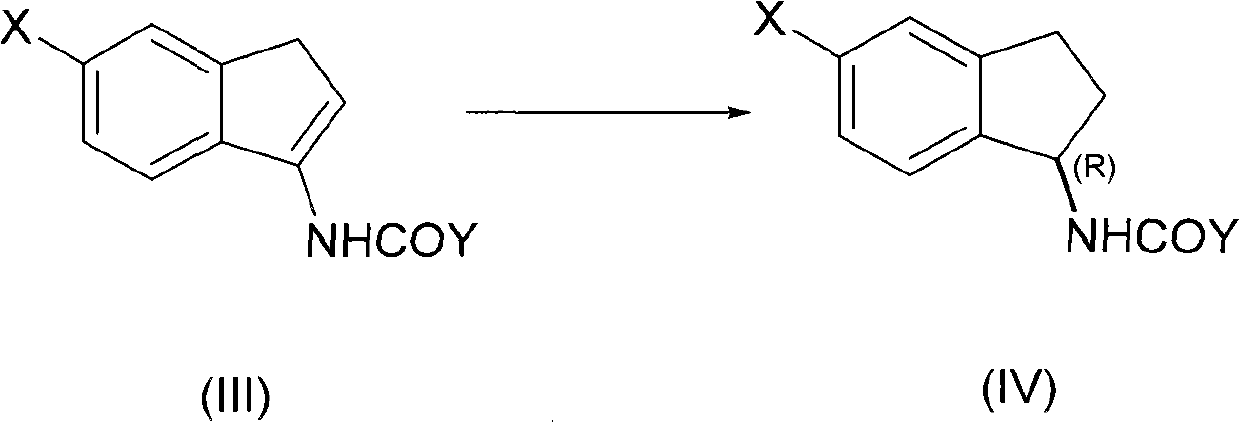
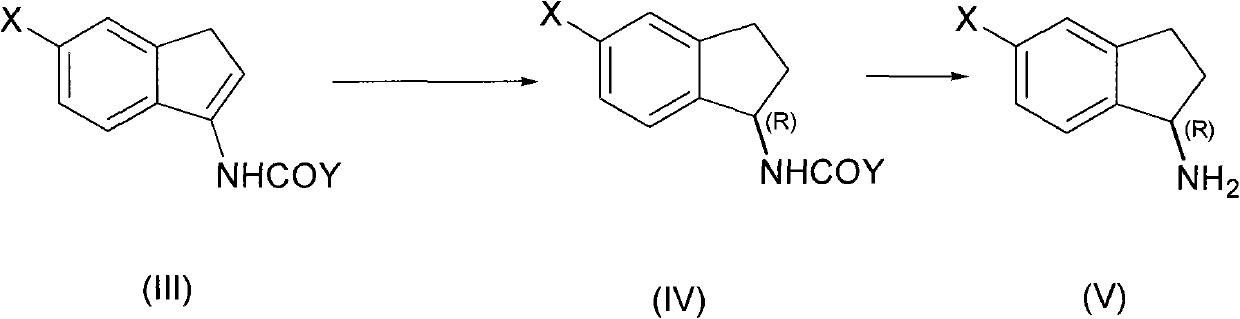
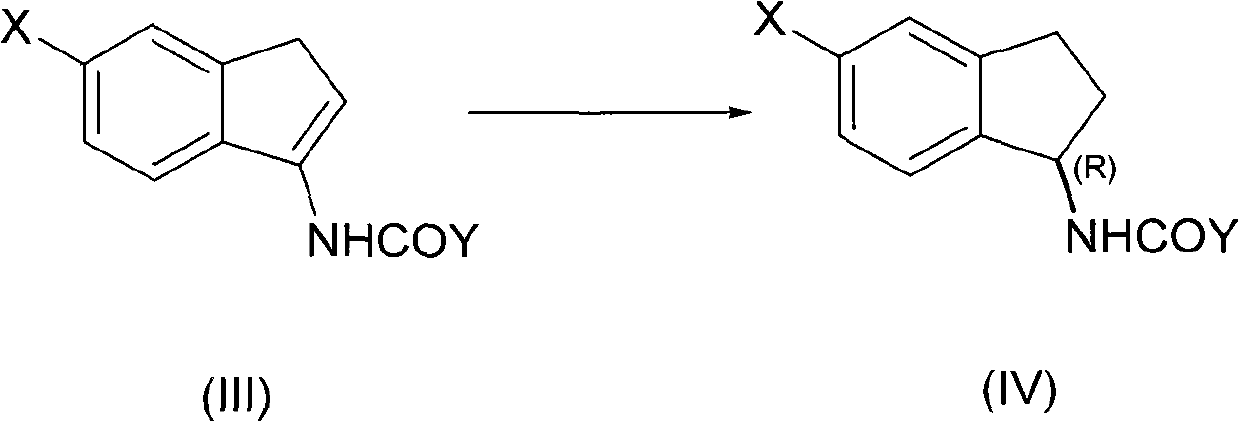
[0041] In a 0.5-liter high-pressure reactor equipped with a thermometer and a stirrer, add acetyl enamine compound (III, X=H, Y=Me) (34.62 g, 0.2 mol) and methanol at room temperature (20-25 ° C). (120 mL), Rh / (RP , Sc)-Duanphos (136 mg, 0.1 mol%), and inject hydrogen to 10 atmospheres. Then heated to 40°C with stirring. HPLC traced the complete reaction of acetylene ss amines (48h). After the reaction, the reaction solution was concentrated and evaporated to remove the organic solvent to obtain (R)-1-acetylindenamine with a yield of 93%, a chemical purity of 99.7%, and an optical purity of 99.8% (HPLC). Then hydrolyze in aqueous sodium hydroxide solution, extract the aqueous phase with dichloromethane organic solvent, concentrate and evaporate the organic solvent, and distill under reduced pressure to obtain (R)-1-indenamine with a yield of 90%, a chemical purity of 99.4%, and an optical Purity 99.8% (HPLC). Using the obtained (R)-1-indenamine as a raw material, react with...
Embodiment 2
[0043] In a 0.5-liter high-pressure reactor equipped with a thermometer and a stirrer, add propionyl enamine compound (III, X=H, Y=Et) (37.44g, 0.2mol) at room temperature (20-25°C), three Fluoroethanol (150 mL), Rh / (Rp, Sc)-Duanphos (68 mg, 0.05 mol%), and hydrogen was injected to 15 atm. Then heated to 60°C with stirring. The reaction of propionyl enamines was followed by HPLC (30h). After the reaction, the reaction liquid was concentrated and evaporated to remove the organic solvent to obtain (R)-1-propionylindamide with a yield of 95%, a chemical purity of 99.8%, and an optical purity of 99.9% (HPLC). Then hydrolyze in aqueous potassium hydroxide solution, extract the aqueous phase with dichloromethane organic solvent, concentrate and evaporate the organic solvent, and distill under reduced pressure to obtain (R)-1-indenamine with a yield of 92%, a chemical purity of 99.7%, and an optical Purity 99.6% (HPLC).
Embodiment 3
[0045] In a 0.5-liter pressure reactor equipped with a thermometer and a stirrer, add acyl enamine compound (III, X=F, Y=Et) (41.04 g, 0.2 mol), tetrahydrofuran ( 180 mL), Rh / (Rp, Sc)-Duanphos (136 mg, 0.1 mol%), and inject hydrogen to 1 atmosphere. Stir at room temperature (20° C.) until the reaction of the propionyl enamine compound is complete (96 h) as tracked by HPLC. After the reaction, the reaction liquid was concentrated and evaporated to remove the organic solvent to obtain the corresponding (R)-5-fluoro-1-propionylindamide with a yield of 90%, a chemical purity of 99.4%, and an optical purity of 99.7% (HPLC). Then hydrolyze in aqueous sodium hydroxide solution, extract the aqueous phase with dichloromethane organic solvent, concentrate and evaporate the organic solvent, and distill under reduced pressure to obtain 5-fluoro-1-indenamine with a yield of 85%, a chemical purity of 99.1%, and an optical Purity 99.6% (HPLC).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com