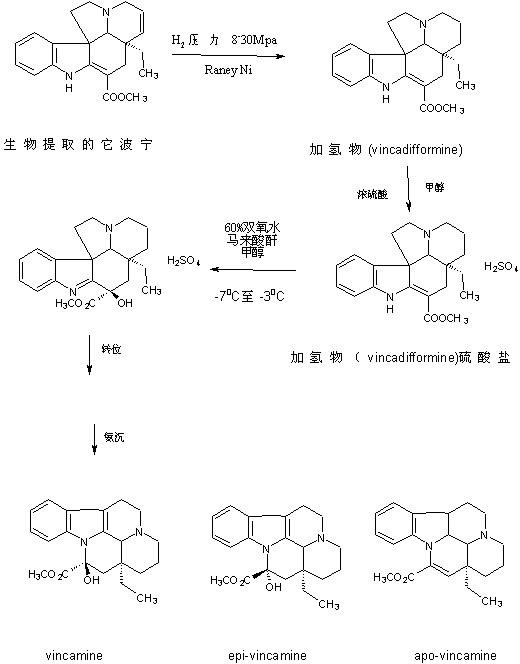
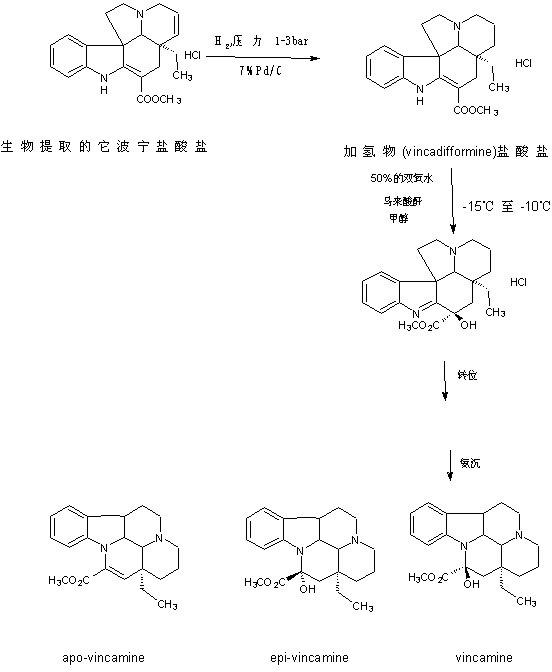
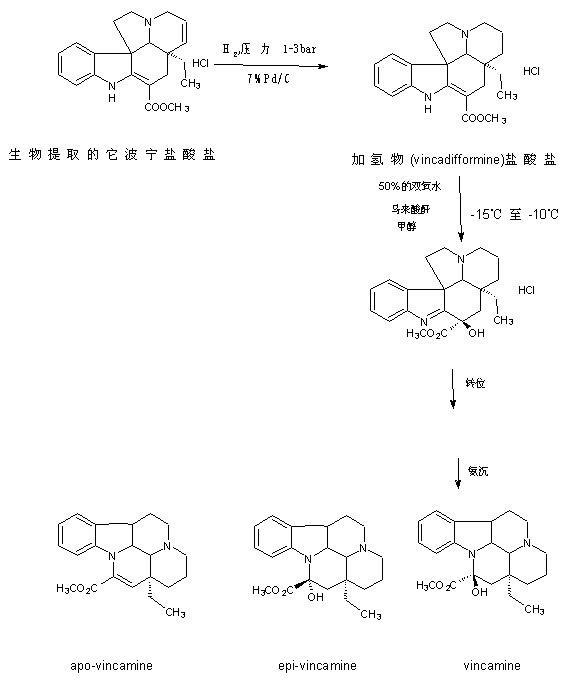
The preparation technology of semi-synthetic vincamine
A preparation process, the technology of vincamine, applied in the field of chemical drug synthesis process, can solve the problems of reducing the ratio of isomer formation, easy loss of activity, easy to burn, etc., and achieve the effect of reducing the amount of addition, reducing cost and reducing pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Preparation of Tabonine Hydrochloride:
[0048] In a 100L sealed enamel reactor, add 50Kg of industrial methanol, after starting the stirring, add 5Kg (13.42mol) of Tabonine hydrochloride, quickly add 1Kg (Tabonine hydrochloride quality 20 The mass percentage of pure Pd is 7% Pd / C (the manufacturer is Shaanxi Ruike New Materials Co., Ltd.), then the lid is sealed, replaced with 99.9% nitrogen for three times, and then hydrogen (purity ≥99.5 %) After replacing the hydrogen for three times, pass hydrogen to a pressure of 0.25Mpa, slowly increase the temperature to 25°C, and stir the reaction at a speed of 100r / min. After reacting for 10 hours, take a sample from the sampling hole and analyze with a TLC dot plate. The volume of the developing agent The ratio is ethyl acetate:petroleum ether=2:3, the amount is 5ml, under the ultraviolet lamp, observe with the wavelength of 254nm, until there is no raw material Tappening hydrochloride. After the reaction, the Pd / C is filter...
Embodiment 2
[0061] 1. Preparation of Tabonine Hydrochloride:
[0062] In a 100L stainless steel reactor, add 50Kg of industrial methanol, after starting the stirring, add 5Kg (13.42mol) of Tabonine hydrochloride, and quickly add 0.5Kg (Tabonine hydrochloride mass 10% ) Newly prepared 7% Pd / C, then add 1.5Kg wet weight in Example 1 to collect the sealed recovered Pd / C, seal the lid of the kettle, replace it with high-purity nitrogen three times, and then use hydrogen (purity ≥99.5% After replacing the hydrogen gas of) for three times, the pressure of hydrogen gas to the pressure gauge on the reactor is 0.35 Mpa, the temperature is slowly raised to 30℃, and the reaction is stirred. After 15 hours of reaction, samples are taken from the sampling hole and analyzed by TLC dot plate. The developing agent is acetic acid Ethyl: Petroleum ether = 2:3, under ultraviolet light, observe with a wavelength of 254nm, until there is no raw material Tabonine hydrochloride. After the reaction, filter Pd / C, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com