Polyaniline nanometer strip compound and preparation method thereof
A technology of polyaniline and composites, applied in the field of materials, can solve the problems that polyaniline has not been reported, and achieve the effects of saving materials and energy, simplifying the preparation process, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of polyacid-doped polyaniline / silver nanoribbon composites:
[0022] Do this in an 80ml glass container. Use a pipette to remove 0.1 ml of aniline liquid, 0.040-0.10 g of silver nitrate and 0.3-0.4 g of H 4 SiW 12 o 40 The acid was dissolved in 40 ml of deionized water and stirred well. Then add 0.3-0.4 grams of ferric nitrate into the above solution, stir quickly and evenly, and then put it into the freezer of the refrigerator at -10°C-18°C for polymerization. After the product turns from light yellow to dark green, thaw the product, filter the resulting dark green product, wash with distilled water and absolute ethanol multiple times to remove impurity ions and oligomers, and vacuum dry it in a vacuum oven at 50°C for 24 hours. use.
[0023] To the characterization of embodiment 1 complex:
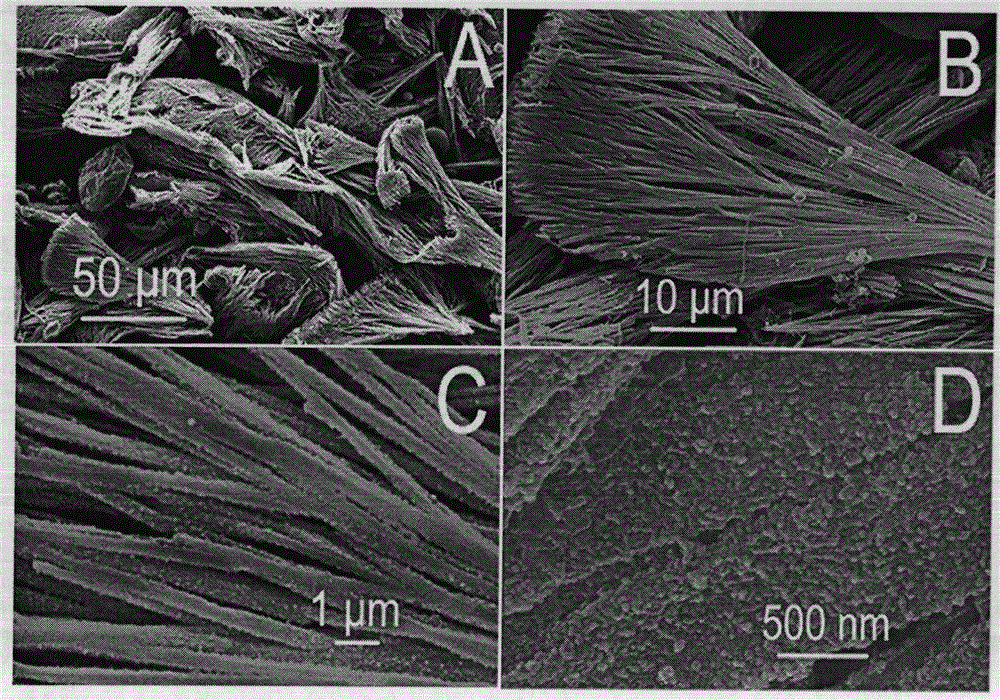
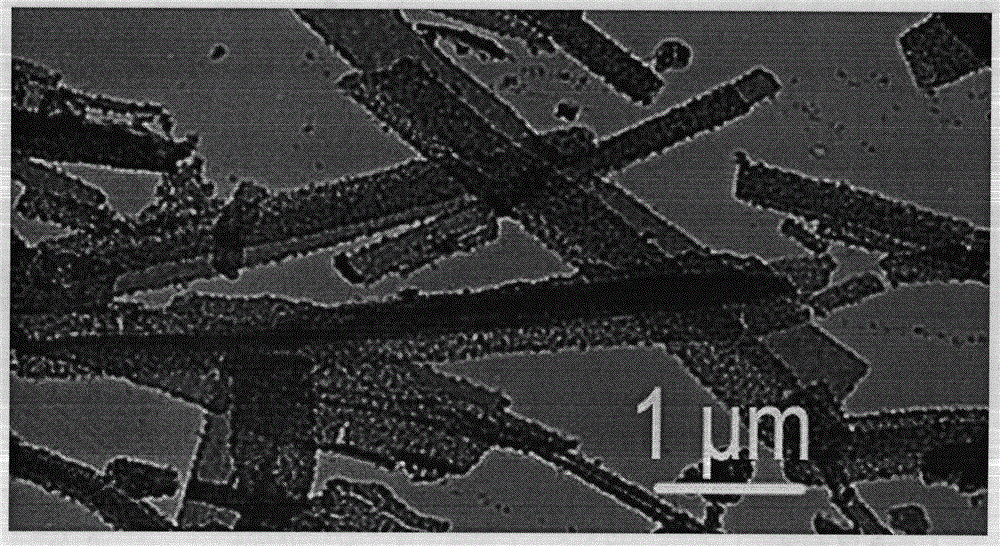
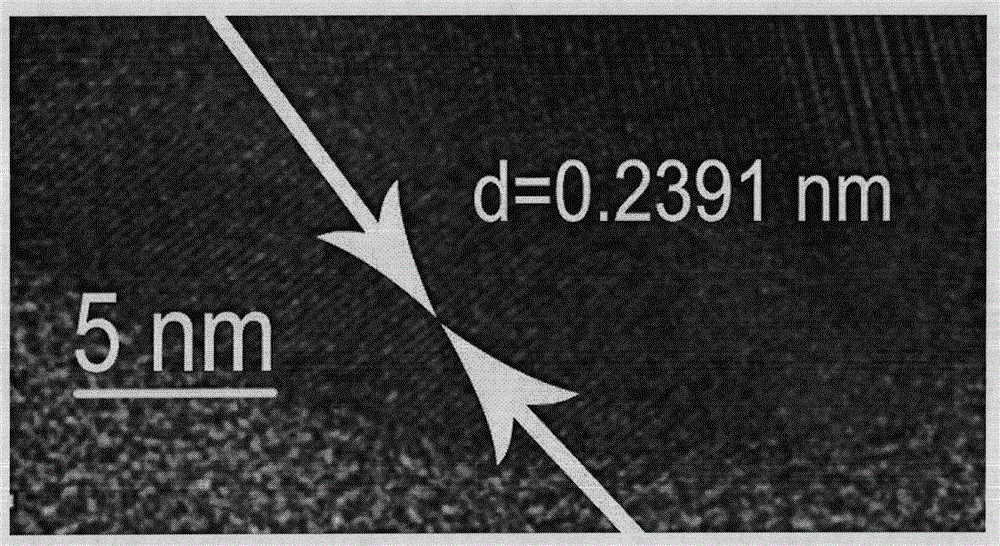
[0024] (1) Scanning electron microscope to characterize the product form
[0025] It was observed with a Hitachi XL-30ESEM FEG scanning electron microscope with a...
Embodiment 2
[0039] Synthesis of polyacid-doped polyaniline / silver nanoribbon composites:
[0040] Do this in an 80ml glass container. Use a pipette to remove 0.1 ml of aniline liquid, 0.04 g of silver nitrate and 0.4 g of H 4 SiW 12 o 40 The acid was dissolved in 40 ml of deionized water and stirred well. Subsequently, 0.4 g of ferric nitrate was added to the above solution, stirred quickly and evenly, and then put into the freezer of the refrigerator at -10°C for polymerization. After the product turns from light yellow to dark green, thaw the product, filter the resulting dark green product, wash with distilled water and absolute ethanol multiple times to remove impurity ions and oligomers, and vacuum dry it in a vacuum oven at 50°C for 24 hours. use.
[0041] Characterization: Same as Example 1.
Embodiment 3
[0043] Synthesis of polyacid-doped polyaniline / silver nanoribbon composites:
[0044] Do this in an 80ml glass container. Use a pipette to remove 0.1 ml of aniline liquid, 0.06 g of silver nitrate and 0.4 g of H 4 SiW 12 o 40 The acid was dissolved in 40 ml of deionized water and stirred well. Subsequently, 0.3 g of ferric nitrate was added to the above solution, stirred quickly and evenly, and then put into the freezer of the refrigerator at -18°C for polymerization. After the product turns from light yellow to dark green, thaw the product, filter the resulting dark green product, wash with distilled water and absolute ethanol multiple times to remove impurity ions and oligomers, and vacuum dry it in a vacuum oven at 50°C for 24 hours. use.
[0045] Characterization: Same as Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com