CuInS2 quantum dots with water-soluble zinc blende structure, CuInS2/ZnS core shell quantum dots with water-soluble zinc blende structure and preparation method thereof
A core-shell structure and quantum dot technology, applied in the field of compound semiconductor nanomaterial preparation, can solve the problems of limited biological application, inability to effectively realize biocompatibility modification, low fluorescence efficiency of quantum dots, etc. Chemical production, good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In the first step, weigh 22.2mg (0.1mmol) InCl 3 , 10.0mg (0.1mmol) CuCl and 218.5mg (1mmol) mercaptoundecanoic acid were placed in a three-necked flask, and 5ml of PEG-400 was added, under the conditions of magnetic stirring and argon protection, the mixture was heated to make InCl 3 and CuCl were completely dissolved and clarified, and vacuumized for 30 minutes to obtain a mixed precursor solution of In and Cu.
[0032] The second step is to backfill the system with argon, and heat the mixed precursor solution of In and Cu from room temperature to 230 °C at a heating rate of 1 °C / s for 30 minutes, then remove the heat source and cool to room temperature, and then centrifuge and purify to obtain CuInS 2 quantum dots.
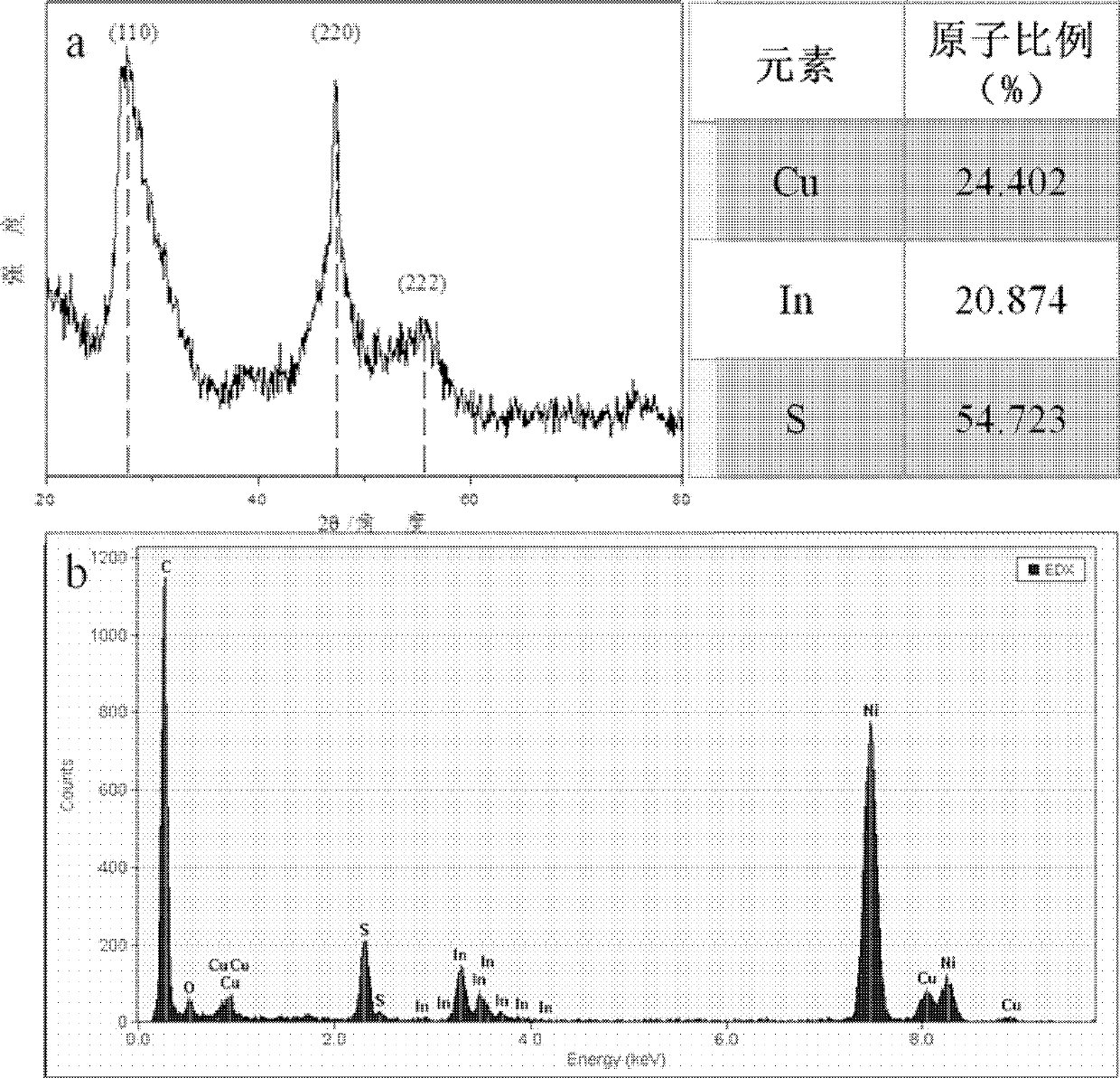
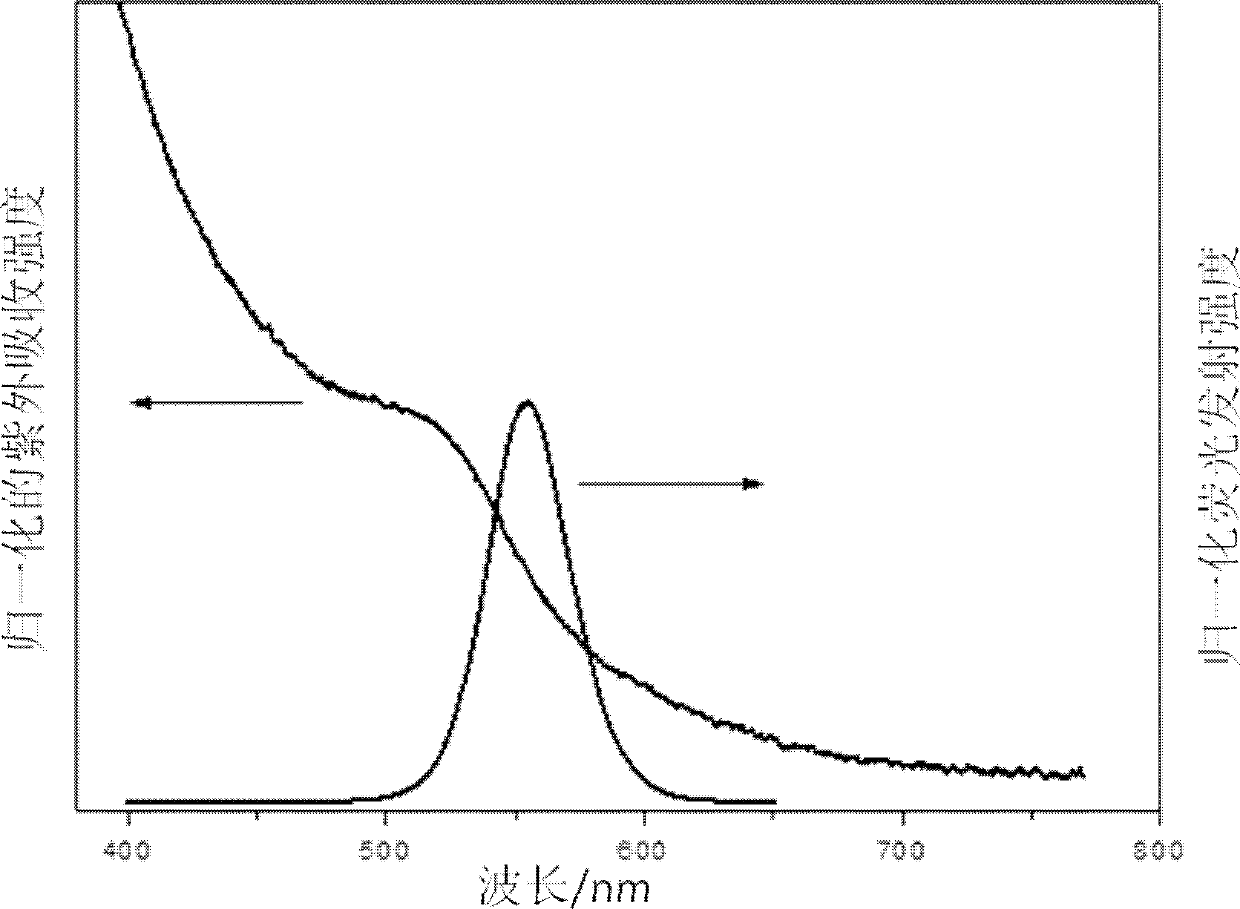
[0033] Such as figure 1 As shown, the obtained CuInS 2 The XRD pattern of quantum dots proves that the obtained nanocrystals are sphalerite structure. The atomic ratio of Cu:In:S was calculated to be 1.17:1:2.62 by electron spectrum analysis of the o...
Embodiment 2
[0035] In the first step, weigh 22.2mg (0.1mmol) InCl 3 , 10.0mg (0.1mmol) CuCl and 218.5mg (1mmol) mercaptoundecanoic acid were placed in a three-necked flask, and 5ml of PEG-400 was added, under the conditions of magnetic stirring and argon protection, the mixture was heated to make InCl 3 and CuCl were completely dissolved and clarified, and vacuumized for 30 minutes to obtain a mixed precursor solution of In and Cu.
[0036] In the second step, argon is backfilled into the system, and the mixed precursor solution of In and Cu is heated from room temperature to 200° C. for 15 minutes at a heating rate of 1° C. / s, and then the third step is performed.
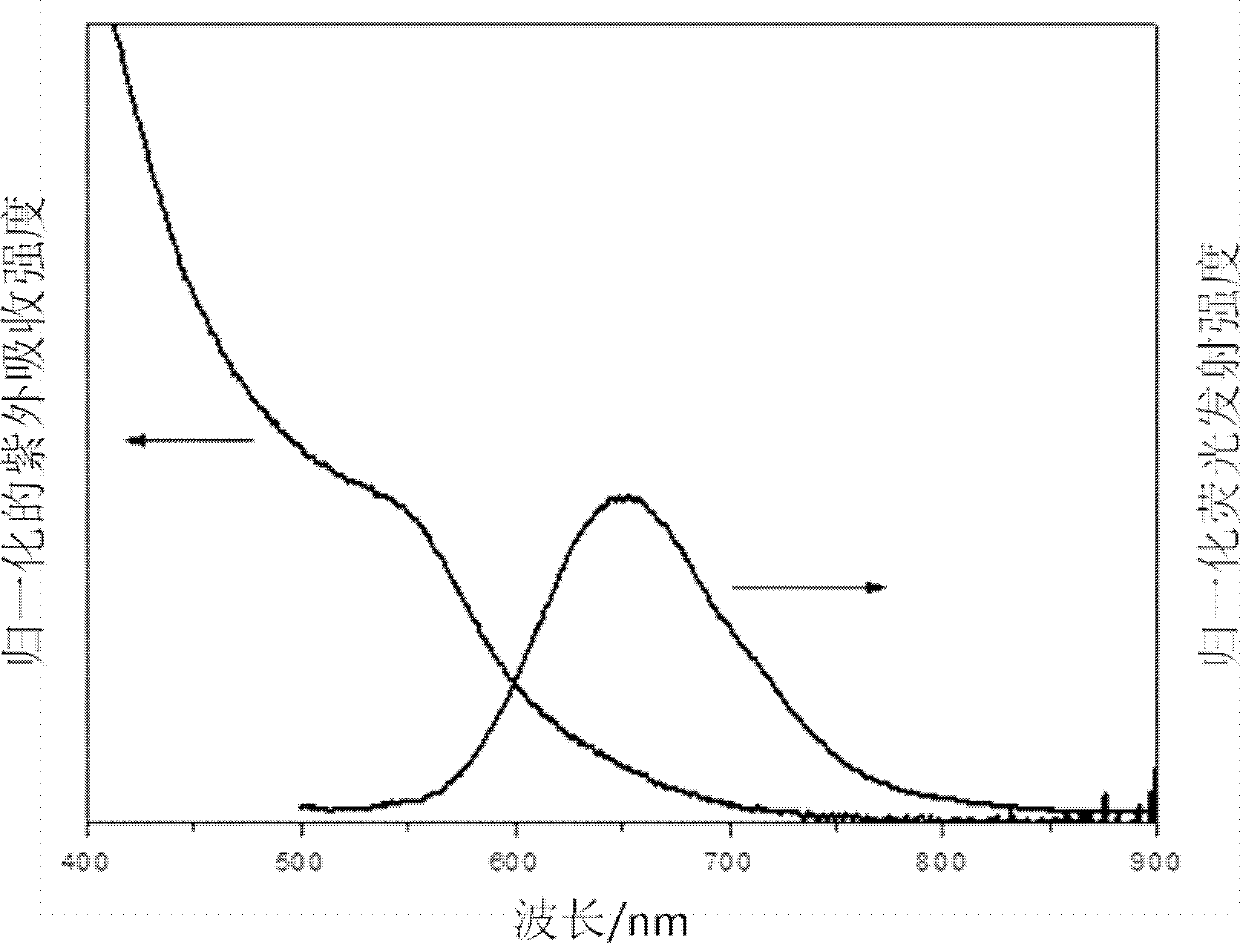
[0037] In the third step, the reaction temperature was adjusted to 220°C, and then Zn(SA) with a concentration of 0.1M was added dropwise 2 -PEG400 solution, after heating and reacting for 30min, remove the heat source and naturally cool to room temperature and centrifuge to obtain water-soluble sphalerite structure CuInS 2...
Embodiment 3
[0040] In the first step, weigh 22.2mg (0.1mmol) InCl 3 , 10.0mg (0.1mmol) CuCl and 437.5mg (2mmol) mercaptoundecanoic acid were placed in a three-necked flask, and 5ml of PEG-800 was added, under the conditions of magnetic stirring and argon protection, the mixture was heated to make InCl 3 and CuCl were completely dissolved and clarified, and vacuumized for 30 minutes to obtain a mixed precursor solution of In and Cu.
[0041] In the second step, argon is backfilled into the system, and the mixed precursor solution of In and Cu is heated from room temperature to 230° C. for 40 minutes at a heating rate of 1° C. / s, and then the third step is performed.
[0042] In the third step, the reaction temperature was adjusted to 220°C, and then Zn(SA) with a concentration of 0.1M was added dropwise 2 -PEG400 solution, after heating and reacting for 30min, remove the heat source and naturally cool to room temperature and centrifuge to obtain water-soluble sphalerite structure CuInS 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com