Thiophene disilole, derivatives thereof and preparation method and use of thiophene disilole and derivatives thereof
A derivative, bisthiophene technology, applied in the application field of thienodithiophene and its derivatives, can solve the problems of difficulty in forming a plane, affecting the molecular band gap, etc., and achieve excellent carrier transport performance and solubility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
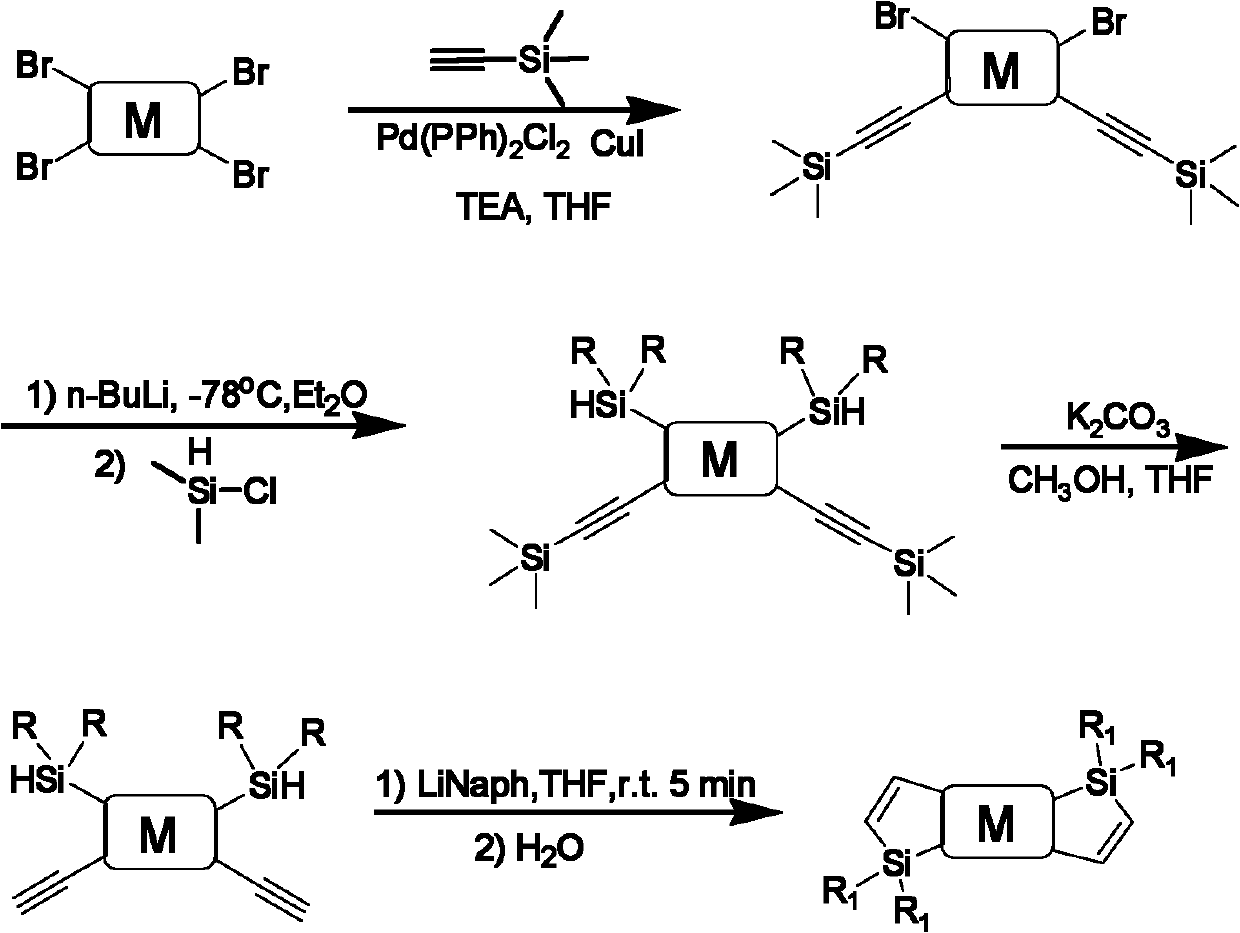
[0043] Si-methyldithiarolothiophene, its chemical structure is:
[0044]
[0045] The preparation method of above-mentioned Si-methyl dithiarolothiophene is as follows:
[0046] In a nitrogen atmosphere, 1 part of tetrabromothiophene solid product and 2.1 parts of trimethylsilylacetylene were dissolved in 300 parts of tetrahydrofuran, 60 parts of triethylamine, 0.05 part of bis(triphenylphosphine)palladium chloride, and 0.1 part of cuprous iodide , and stirred at reflux for 12 hours to obtain a dibromothiophene compound of trimethylsilylacetylene;
[0047] Dissolve 1 part of dibromothiophene compound of trimethylsilylacetylene in 300 parts of ether, cool to -78°C, add 2.5 mol / L n-hexane solution of butyllithium, and add 2.1 parts of dialkyl chloride after 1 hour Silane, warming up to room temperature, stirring for 24 hours to obtain a substituted thiophene compound of trimethylsilylacetylene and dialkylsilyl;
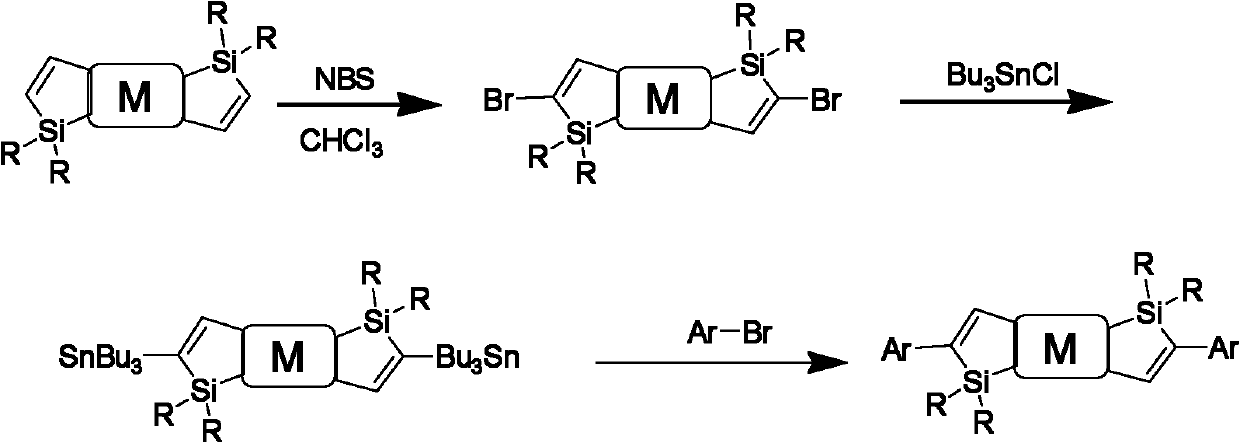
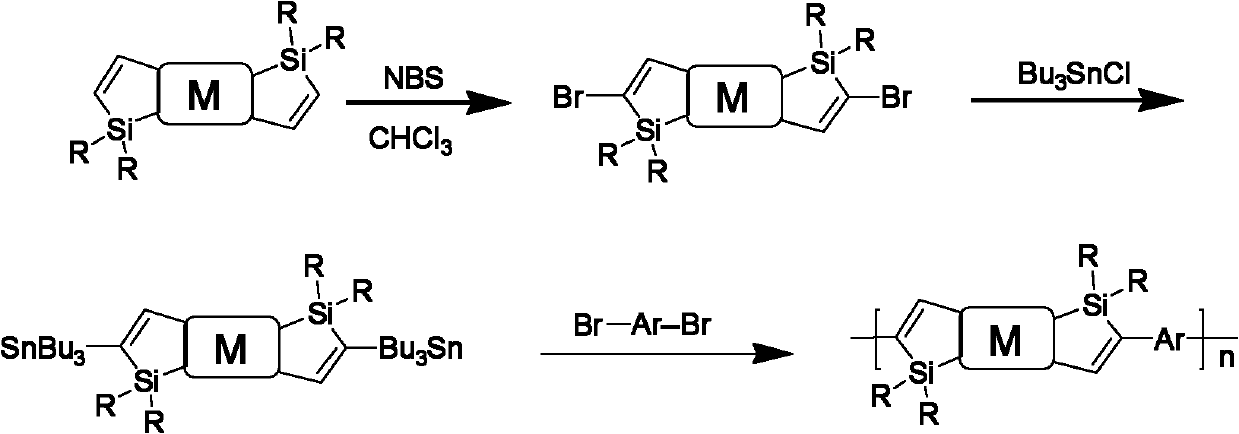
[0048] Dissolve 1 part of trimethylsilylacetylene and dialkyl...
Embodiment 2
[0051] Si-methyldithiarolodithiophene, its chemical structure is:
[0052]
[0053] The preparation method of above-mentioned Si-methyl dithiarodithiophene is as follows:
[0054] In a nitrogen atmosphere, 1 part of tetrabromodithiophene solid product and 2.1 parts of trimethylsilylacetylene were dissolved in 300 parts of tetrahydrofuran, 60 parts of triethylamine, 0.05 part of bis(triphenylphosphine) palladium chloride, 0.1 part of iodide Cuprous, refluxed and stirred for 12 hours, obtained the dibromodithiophene compound of trimethylsilylacetylene;
[0055] Dissolve 1 part of dibromodithiophene compound of trimethylsilylacetylene in 300 parts of ether, cool to -78°C, add 2.5 mol / L butyllithium in n-hexane, and add 2.1 parts of dioxane after 1 hour Chlorosilane, warming up to room temperature, stirring for 24 hours, to obtain trimethylsilyl acetylene and dialkylsilyl substituted dithiophene compound;
[0056] Dissolve 1 part of trimethylsilylacetylene and dialkylsilyl-di...
Embodiment 3
[0059] Si-methyldithiarolotrithiophene, its chemical structure is:
[0060]
[0061] The preparation method of above-mentioned Si-methyl dithiarolotrithiophene is as follows:
[0062] In a nitrogen atmosphere, 1 part of tetrabromotrithiophene solid product and 2.1 parts of trimethylsilylacetylene were dissolved in 300 parts of tetrahydrofuran, 60 parts of triethylamine, 0.05 part of bis(triphenylphosphine) palladium chloride, 0.1 part of iodide Cuprous, refluxed and stirred for 12 hours, obtained the dibromotrithiophene compound of trimethylsilylacetylene;
[0063] Dissolve 1 part of the dibromotrithiophene compound of trimethylsilylacetylene in 300 parts of ether, cool to -78°C, add 2.5 mol / L butyllithium in n-hexane, and add 2.1 parts of dioxane after 1 hour Chlorosilane, warming up to room temperature, stirring for 24 hours to obtain trimethylsilyl acetylene and dialkylsilyl substituted trithiophene compound;
[0064] Dissolve 1 part of trimethylsilylacetylene and dial...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com