A kind of respiratory syncytial virus f2 protein subunit vaccine and preparation method thereof
A subunit vaccine and syncytial virus technology, applied in the field of respiratory syncytial virus F2 protein subunit vaccine and preparation thereof, can solve the problems of relying on cell culture to obtain, unfavorable for vaccine popularization, and high environmental requirements, and achieves easy culture and fermentation, Simple structure and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Obtaining of F2 gene
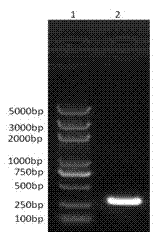
[0026] Amplify the F / 18T / DH5α strain (preserved in our laboratory, containing the full gene of RSV fusion protein F), and then use the plasmid extraction kit from Biotech to extract the plasmid (the following plasmids are extracted according to the instructions), so as to The plasmid was used as a template, and the F2 gene was amplified with TIANGEN’s Taq DNA polymerase, and the Nco I, Bam HI two restriction sites and 6×His tag, the primer sequence used is: F2PFNP1: 5'-CCGCCATGGAGGCTTCCAGTCAAAAACATCACTGAAGAATTT-3', F2PFNP2: 5'-CGCGGATCCTTAGTGGTGGTGGTGGTGGTGCCTTCTGGCTCGATTGTTGGCTG-3'; PCR amplification conditions: 94°C 5min; 94 ℃ for 30s, 50℃ for 30s, 72℃ for 30s (30 cycles); 72℃ for 5min; the amplified product was recovered and identified by 1% agarose gel electrophoresis, and the F2 gene with a size of 290bp was amplified (see figure 1 ).
Embodiment 2
[0027] Example 2 Recombinant expression vector F2 / pFN18K and Recombinant engineering bacteria F2 / pFN18K / KRX Construct
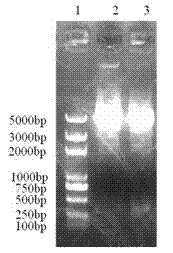
[0028] The F2 gene was purified using the Gel Recovery Kit from Biotech (the following gel recovery and purification were performed according to the instruction manual) and the pFN18K plasmid was extracted using the small amount of plasmid extraction kit from Biotech (purchased from Promega, USA). Nco I and Bam H Ⅰ (purchased from TAKARA company) at 37°C for double digestion of F2 gene and pFN18K plasmid for 3 hours. Recover the double-digested F2 fragment and pFN18K fragment with a gel recovery kit. Take 180ng of F2 fragment and 90ng of pFN18K fragment, use 0.5μl T4 DNA ligase (from TAKARA company, CK5021B) to connect overnight at 16°C, and transform the ligation product by heat shock method with CaCl 2 Prepared E. coli KRX Competent Cells (purchased from Promega, USA), coated with Kan + -LB plate, cultivated at 37°C for 16 hours, picked several s...
Embodiment 3
[0029] Example 3 Induced expression of Halo-F2 protein
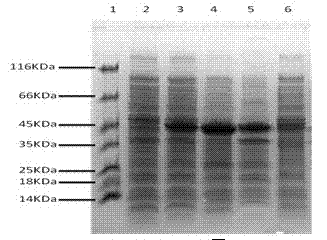
[0030] Pick a single colony of recombinant engineering bacteria F2 / pFN18K / KRX and inoculate it into Kan + -In LB culture medium, cultivate overnight at 37°C with shaking, and inoculate fresh Kan at 1% the next day + -In LB culture medium, shake culture at 37°C until OD600 is about 0.8, take appropriate samples and add rhamnose (sourced from Sanland-chem International Inc, 102189) at a final concentration of 1 μl / ml respectively, and induce expression at 28°C Overnight, after the end of the induction, take an appropriate amount of samples for SDS-PAGE to determine whether the size is correct. The apparent molecular weight of the Halo-F2 protein is 13.3KDa (see image 3 ), which is basically consistent with the theoretical calculation value, expressed in two forms of inclusion body and soluble protein.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com