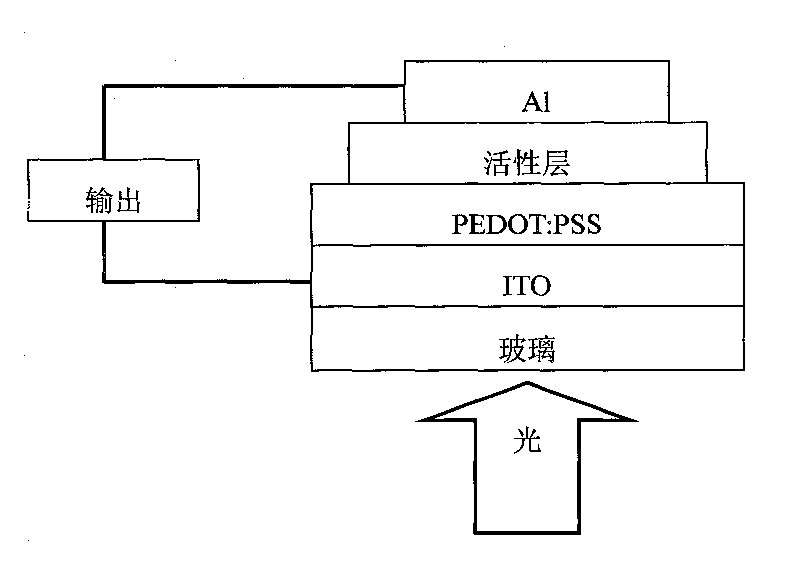
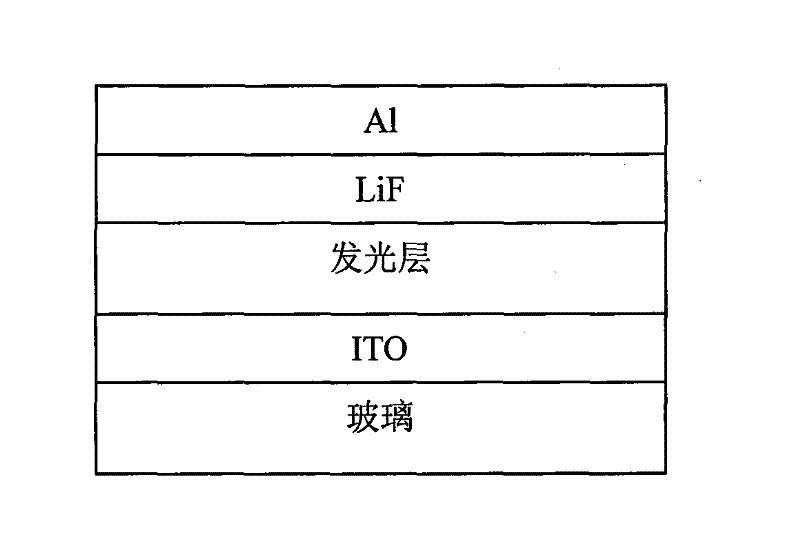
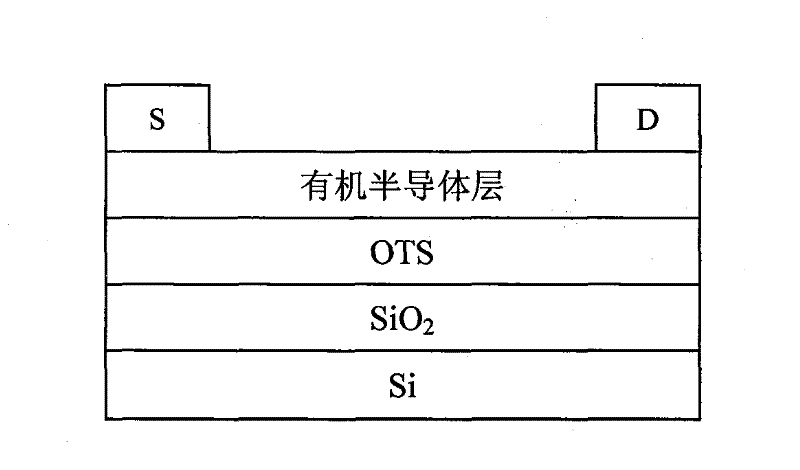
Carbazole-containing porphyrin-thienothiadiazole copolymer and its preparation method and application
A technology of carbazole porphyrin and alkyl carbazole porphyrin, applied in the field of copolymers, can solve the problems such as no literature and patent reports, limited application scope, etc., and achieve the effects of improving solubility, expanding application scope and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] A kind of preparation method containing carbazole porphyrin-thienothiadiazole copolymer, comprises the steps:
[0028] 5,15-dibromo-10,20-alkylcarbazole porphyrins of structural formula (A) and bisboronate-thieno[3,4-c][1,2,5] of structural formula (B) are provided Thiadiazoles:
[0029]
[0030] In the formula: n is an integer between 1-100, R 1 , R 2 for the same or different hydrogen or C 1 -C 32 of alkyl.
[0031] In an oxygen-free environment (the present invention uses nitrogen and / or inert gas as protective gas to form an oxygen-free environment), under the presence of a catalyst and an organic solvent, 5,15-dibromo-10,20-alkylcarbazoleporphyrin Carry out Suzuki coupling reaction with double boronate group thieno[3,4-c][1,2,5]thiadiazole, obtain the described carbazole-containing porphyrin-thienothiadiazole copolymerization of structural formula (I) The solution of the substance; wherein, the molar dosage of the catalyst is 0.05%-30% of the bisboronate-b...
Embodiment 1
[0037] This embodiment discloses a 10,20-bis(9-octylcarbazole) porphyrinthieno[3,4-c][1,2,5]thiadiazole copolymer as an organic semiconductor material, its structural formula as follows:
[0038]
[0039] In the formula: n is an integer between 1-100.
[0040]The preparation steps of this copolymer are as follows:
[0041] 1. 5,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane)thieno[3,4-c][1,2,5]thio Synthesis of Oxadiazole
[0042]
[0043] Under the protection of nitrogen, 8.8 g (0.03 mol) of 5,7-dibromothieno[3,4-c][1,2,5]thiadiazole was added to the three-necked flask, and 200 ml of tetrahydrofuran solvent was added 25.2 mL (0.06 mol) of n-butyllithium with a concentration of 2.5M was slowly injected with a syringe at -78°C, and after stirring for 2 hours, 2-isopropoxy was injected with a syringe at -78°C Base-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 13 mL (ie. 06 mol), warmed up to room temperature and stirred overnight. Then add saturated 30ml sodium chloride aque...
Embodiment 2
[0058] This embodiment discloses a 10-(9-methylcarbazole)-20-(9-docosylcarbazole) porphyrinthieno[3,4-c][1,2 , 5] the copolymer of thiadiazole, its structural formula is as follows:
[0059]
[0060] In the formula: n is an integer between 1-100.
[0061] The preparation steps of this copolymer are as follows:
[0062] 1. 5,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) thieno[3,4-c][1,2,5] Synthesis of Thiadiazoles
[0063] See Example 1 for its preparation.
[0064] Two, the synthesis of 5-(9-methylcarbazole)-15-(9-docosylcarbazole) porphyrin
[0065]
[0066] Set up an anhydrous and oxygen-free device, weigh the intermediate 2-aldehyde-9-methylcarbazole (0.21g, 1mmol), 2-aldehyde-9-docosylcarbazole 0.65g (ie 1mmol), di Dissolve 0.30 g (ie 2 mmol) of pyrromethane in 250 ml of dichloromethane, blow nitrogen gas for 30 min, add 2 ml of trifluoroacetic acid into a syringe, stir at 100 °C for 1 h, then add 1.82 g of dichlorodicyanobenzoquinone (DDQ) ( 8mmol), continu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet resistance | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com