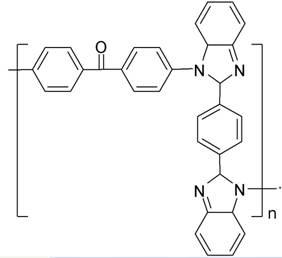
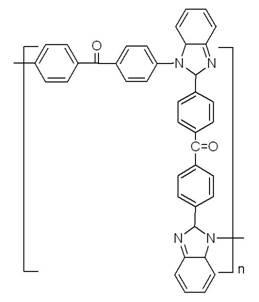
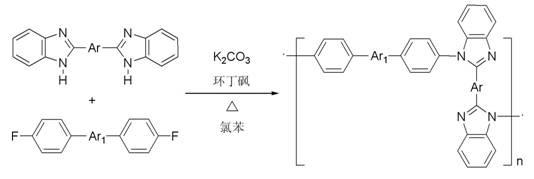
Polyarylketone imidazole and its preparation method
A technology for polyarylketimidazole and bisbenzimidazole is applied in the field of polyarylketimidazole and its preparation, and can solve the problems of harsh operating conditions, complex synthesis process routes, serious environmental pollution and the like, and achieves low preparation cost and simple operation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 50mL three-necked flask, add 0.01mol terephthalic acid, 0.022 o-phenylenediamine, 2mL polyphosphoric acid and 8mL phosphoric acid, under N 2 Under the protection of , the reaction was stirred at 190 °C for 4 h. After the solution is cooled, pour it into 100 mL of cold distilled water, add 15% NaOH solution dropwise, neutralize it to pH=8, and generate a large amount of light green precipitates. After standing for 6 h, the solution was filtered, washed with water and dried to obtain a solid crude product. Dissolve the crude product in N,N- Dimethylformamide was recrystallized and vacuum dried at 60°C to obtain the bisbenzimidazole intermediate- .
[0026]
[0027] Under a nitrogen atmosphere, the bisbenzimidazole intermediate- 0.01mol, 0.01mol of 4'4-difluorobenzophenone, 0.02mol of anhydrous potassium carbonate, 7mL of sulfolane and 3mL of chlorobenzene. The system was raised from room temperature to 145 °C for 2 h. Then the temperature is raised from ...
Embodiment 2
[0031] In a 50mL three-necked flask, add 0.01mol 4'4 diacid diphenyl ether, 0.022 o-phenylenediamine, 2mL polyphosphoric acid and 8mL phosphoric acid, under N 2 Under the protection of , the reaction was stirred at 190 °C for 4 h. After the solution was cooled, pour it into 100 mL of cold distilled water, add 15% NaOH solution dropwise, and neutralize it to pH=8, resulting in a large amount of green precipitates. After the solution was placed for 6 hours, suction filtration, washing with water, and drying were performed to obtain a solid crude product. The crude product was dissolved in absolute ethanol for recrystallization and vacuum drying at 60 °C to obtain the bisbenzimidazole intermediate- .
[0032]
[0033] Under a nitrogen atmosphere, the bisbenzimidazole intermediate- 0.01mol, 0.01mol of 4'4-difluorobenzophenone, 0.02mol of anhydrous potassium carbonate, 7mL of sulfolane and 3mL of chlorobenzene. The system was raised from room temperature to 145 °C for 2 h...
Embodiment 3
[0037] In a 50mL three-neck flask, add 0.01mol 4'4 diacid benzophenone, 0.022 o-phenylenediamine, 2mL polyphosphoric acid and 8mL phosphoric acid, in N 2 Under the protection of , the reaction was stirred at 190 °C for 4 h. After the solution was cooled, pour it into 100 mL of cold distilled water, add 15% NaOH solution dropwise, and neutralize it to pH=8, resulting in a large amount of green precipitates. After the solution was placed for 6 hours, suction filtration, washing with water, and drying were performed to obtain a solid crude product. Dissolve the crude product in N,N - Dimethylformamide (DMF) was recrystallized and vacuum dried at 60°C to obtain the bisbenzimidazole intermediate - .
[0038]
[0039]
[0040] Under a nitrogen atmosphere, the bisbenzimidazole intermediate- 0.01mol, 0.01mol of 4'4-difluorobenzophenone, 0.02mol of anhydrous potassium carbonate, 7mL of sulfolane and 3mL of chlorobenzene. The system was raised from room temperature to 145 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com