Fullerene galactoside derivative, and preparation method and application thereof
A technology of fullerene and derivatives, applied in the field of fullerene galactose derivatives, can solve the problems of impurity, sample loss, mixed impurities, etc., and achieve the effect of strong removal of superoxide anion free radicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 The preparation method of fullerene monoaddition and double addition acetylated galactose derivatives
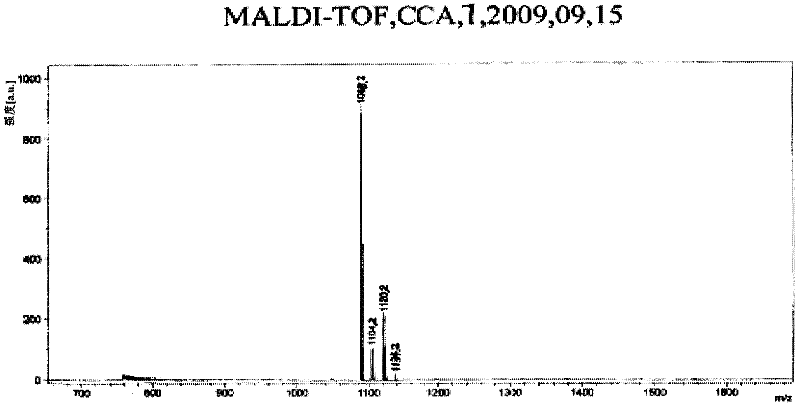
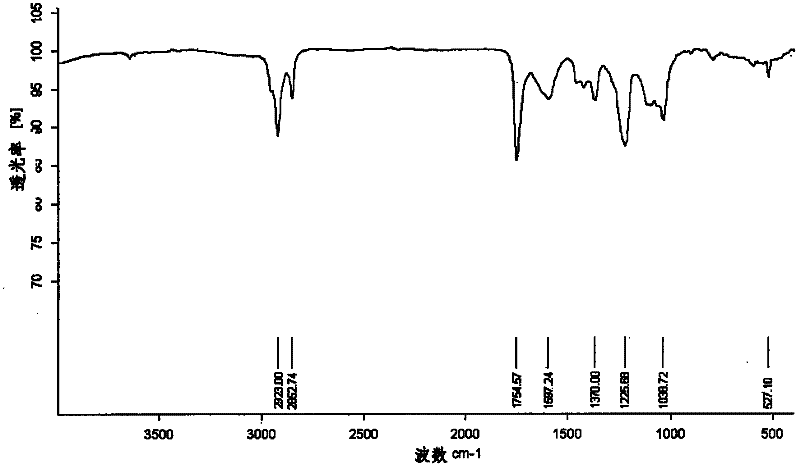
[0038] In a 250ml three-neck round bottom flask, add 56mg 2,3,4,6-O-tetraacetyl-1-azido-beta-D-galactose and 72mg C 60 , 16ml of chlorobenzene, under the protection of argon, the oil bath was refluxed at 131°C for 8 hours. The reaction process was monitored by TLC, and a brown band appeared at Rf=0.6. After the reaction, the solvent was evaporated to dryness, and the solid residue was separated with a silica gel column. When the eluting solution is toluene / ethyl acetate=10 / 1, the monoaddition product of fullerene galactose is obtained; when the eluting solution is toluene / ethyl acetate=5 / 1, the fullerene galactose is obtained The double-addition product; when the eluting solution is toluene, unreacted C 60 . Both mono-addition and double-addition derivatives are brown solids, and the yields are 21.6 mg (16.8%) and 15.6 mg (12.18%), respectively. The ...
Embodiment 2
[0039] Embodiment 2 The preparation method of fullerene monoaddition and double addition acetylated galactose derivatives
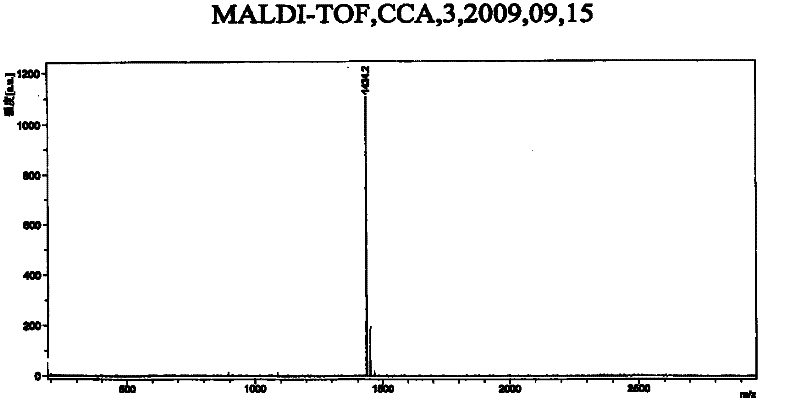
[0040] In a 500ml three-neck round bottom flask, add 112mg 2,3,4,6-O-tetraacetyl-1-azido-beta-D-galactose and 144mg C 60 , 32ml of dichloromethane, refluxed in an oil bath at 120°C for 14 hours under the protection of argon. The reaction process was monitored by TLC, and a brown band appeared at Rf=0.6. After the reaction, the solvent was evaporated to dryness, and the solid residue was separated with a silica gel column. When the eluting solution is toluene / ethyl acetate=10 / 1, the monoaddition product of fullerene galactose is obtained; when the eluting solution is toluene / ethyl acetate=5 / 1, the fullerene galactose is obtained The double-addition product; when the eluting solution is toluene, unreacted C 60 . Both mono-addition and double-addition derivatives are brown solids, and the yields are 41.2 mg (16.09%) and 30.2 mg (11.8%), respectively. Th...
experiment example 1
[0041]Experimental Example 1 Detection of Superoxide Anion Free Radical Scavenging Ability in Pyrogallol Autoxidation System
[0042] 1. Principle
[0043] Pyrogallol can rapidly self-oxidize under alkaline conditions, generate a series of intermediate products with strong absorption at 300-325nm, and release superoxide anion (O 2 - ), to detect the rate of change of absorbance at 320nm, which represents the rate of autoxidation of pyrogallol. If a compound that can scavenge superoxide anion is added to the system, the autoxidation rate of pyrogallol will be slowed down. The autoxidation rate of pyrogallol is accelerated if a compound that can generate superoxide anion is added.
[0044] Sample Clear O 2 - The clearance rate (S) is calculated by the following formula:
[0045] S=(K 0 -K 1 ) / K 0 ×100%
[0046] where K 0 represents the standard autoxidation rate of pyrogallol, K 1 Represents the autoxidation rate of the added sample group.
[0047] 2. Experiment
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com