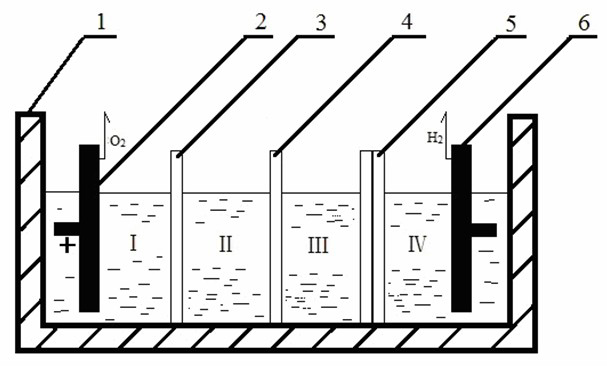
Three-membrane four-chamber chlorine-free alkali-producing electrolytic tank consisting of anion and cation exchange membranes and bipolar membrane
An anion-cation-exchange membrane and anion-exchange membrane technology, applied in the field of electrolyzers, can solve the problems of sharp drop in sales, breakage of chlor-alkali balance, etc., and achieve the effects of increasing hydrophilic properties, increasing alkali production, and improving the efficiency of electrolyzers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of cation exchange membrane:
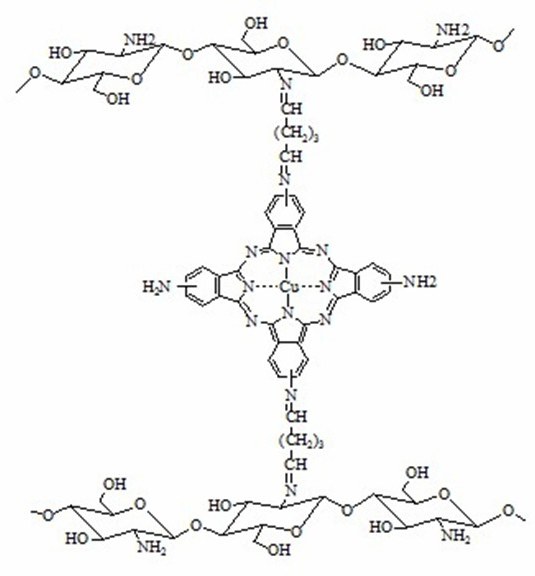
[0027] Accurately weigh 6 g of sodium carboxymethylcellulose (CMC) to prepare 200 mL of CMC aqueous solution, and weigh 0.1 g of tetracarboxyiron phthalocyanine [FePc(COOH) 4 ], dissolved in 10mL tetrahydrofuran, slowly added to the CMC aqueous solution, stirred evenly, and degassed under reduced pressure to obtain a viscous film solution, which was cast on a flat petri dish and air-dried at room temperature to form a film, with a weight fraction of 8% (The same below) soaked in chromium chloride solution for 30min, rinsed with distilled water, and dried naturally to obtain FePc(COOH) 4 - CMC cation exchange membrane.
[0028] Preparation of anion exchange membrane:
[0029] Accurately weigh 6 g of chitosan, dissolve it with 2.0% acetic acid aqueous solution to prepare chitosan acetic acid aqueous solution with a weight fraction of 3.0%, and weigh 0.08 g of tetraaminoiron phthalocyanine [FePc(NH 2 ) 4 ], dissolved in 10 mL ...
Embodiment 2
[0038] Preparation of cation exchange membrane:
[0039] Accurately weigh 4 g of sodium carboxymethylcellulose (CMC) to prepare 280 mL of CMC aqueous solution, and weigh 0.08 g of tetracarboxy copper phthalocyanine [CuPc(COOH) 4 ], dissolved in 10mL tetrahydrofuran, slowly added to the CMC aqueous solution, stirred evenly, and degassed under reduced pressure to obtain a viscous film solution, which was cast on a flat petri dish and air-dried at room temperature to form a film, with a weight fraction of 8% (The same below) soaked in chromium chloride solution for 40min, rinsed with distilled water, and dried naturally to obtain CuPc(COOH) 4 - CMC cation exchange membrane.
[0040] Preparation of anion exchange membrane:
[0041] Accurately weigh 3 g of chitosan, dissolve it with 2.0% acetic acid aqueous solution, and prepare a chitosan acetic acid aqueous solution with a weight fraction of 3.5%, and weigh 0.07 g of tetraaminocopper phthalocyanine [CuPc(NH 2 ) 4 ], dissolved...
Embodiment 3
[0047] The structure of the electrolytic cell is as in Example 1. The cathode electrode of the electrolytic cell is a DSA electrode, and the anode electrode of the electrolytic cell is a lead electrode. The cation exchange membrane is PbPc(COOH) 4 -CM, the anion exchange membrane is Pb Pc(NH 2 ) 4 -CS.
[0048] At 40°C, adjust the cathode and anode current densities to 100mA·cm -2 , After 7 hours of electrolytic dialysis, the concentration of NaOH in the middle chamber is 36.7g / L, and the current efficiency is 65%. The cell voltage is 5.1V.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com