Production method of encephalitis B vaccine
A production method, Japanese encephalitis technology, applied in the field of production of inactivated virus Japanese encephalitis vaccine using bioreactors, can solve the problems of restricting industrial production and clinical application of virus vaccines, unstable product quality, insufficient production capacity, etc. , to achieve the effect of ensuring uniformity and stability, eliminating instability, and increasing cell density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Recovery of VERO cell seeds
[0033] 1.1 Source of VERO cell line
[0034] The original source of VERO cells is ATCC (No. F-12313), and the main cell bank and working cell bank were established after subculture and expansion, and stored in liquid nitrogen. The main cell is 129 passages; the working cell is 133 passages, and the cryopreservation density is 4×10 6 / ml.
[0035] 1.2 Recovery of VERO-seeded cells
[0036] Take one cell of the working bank stored in the liquid nitrogen tank, and after the warm water at 39°C melts rapidly, the cell suspension is divided into about 1×10 5 / cm2 Inoculation density transferred to 175cm 2 In the square bottle, gradually add medium (M199 containing 10% (volume / volume) fetal bovine serum (M199 dry powder medium composition see GIBCO medium manual) dropwise to 60mL, 37 ℃ and 5% CO 2 cultured in an incubator.
[0037] 2. At 175cm 2 Cultivate primary seed cells in square flasks
[0038] The revived cells were cultured for 3...
Embodiment 2
[0126] 1. The batch-to-batch stability research of the product prepared by the production method of the present invention
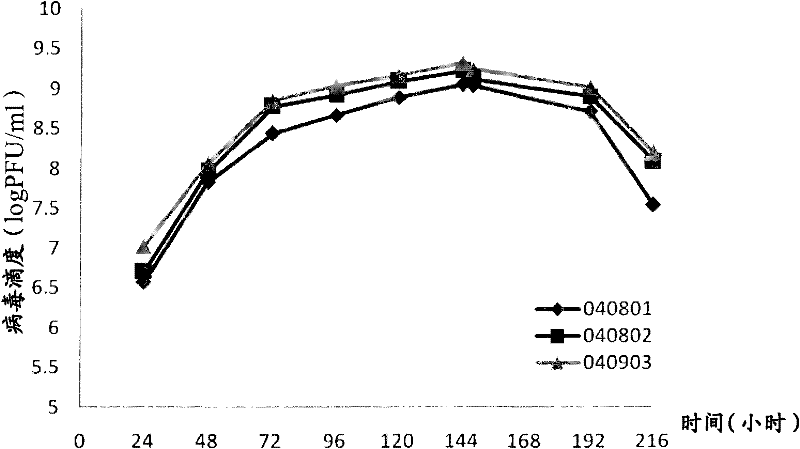
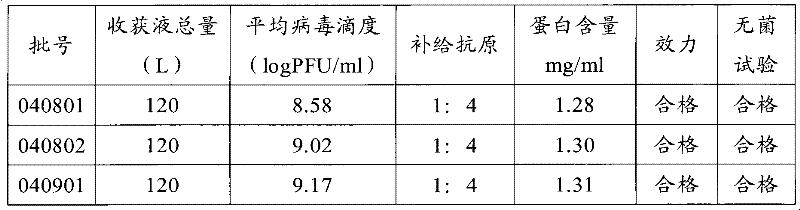
[0127] The detection results of the three batches of pilot culture stock solutions of 040801, 040802 and 040901 showed that there were no significant differences in virus titer, complement antigen, protein residue, sterility test and potency of the three batches of sample harvest liquid (see Table 1).
[0128] Table 1 Test results of three batches of pilot culture harvest liquid
[0129]
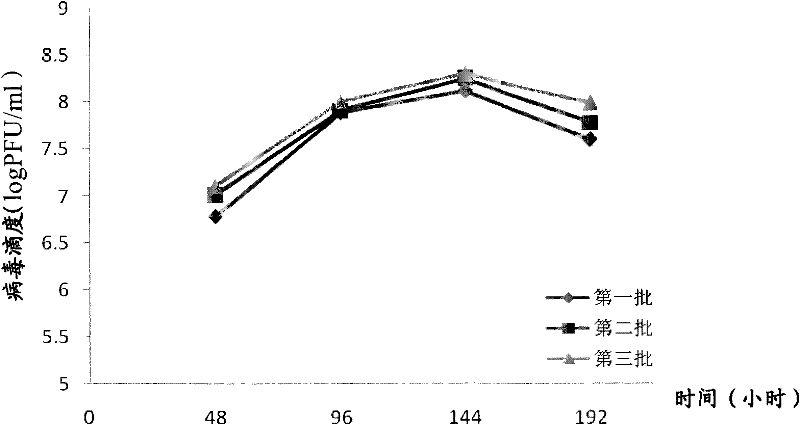
[0130] 2. Research on culturing VERO cells and propagating JE P3 strains in spinner bottles
[0131] VERO cells were subcultured into 2L square bottles after passage in square bottles, and then expanded and cultured at a ratio of 1:4. Three batches of 16 bottles were cultured. The cell culture conditions are as follows: the formulation of the culture medium and maintenance solution is the same as the above 6.1, the temperature is 37°C, and the rotation speed is 1 / 8 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com