Phosphate material for efficiently and selectively removing lead ions and application thereof
A lead ion, selective technology, applied in the field of removal of heavy metal ions in solution, to achieve the effect of small particle size, high purity, and many reactive sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055](1) Dissolve 1.07g of strontium nitrate in 50mL of deionized water, add 0.036g of hexadecyltrimethylammonium bromide, stir thoroughly for 30min to disperse evenly, and adjust the pH with 0.01mol / L dilute nitric acid = 5. Get a solution.
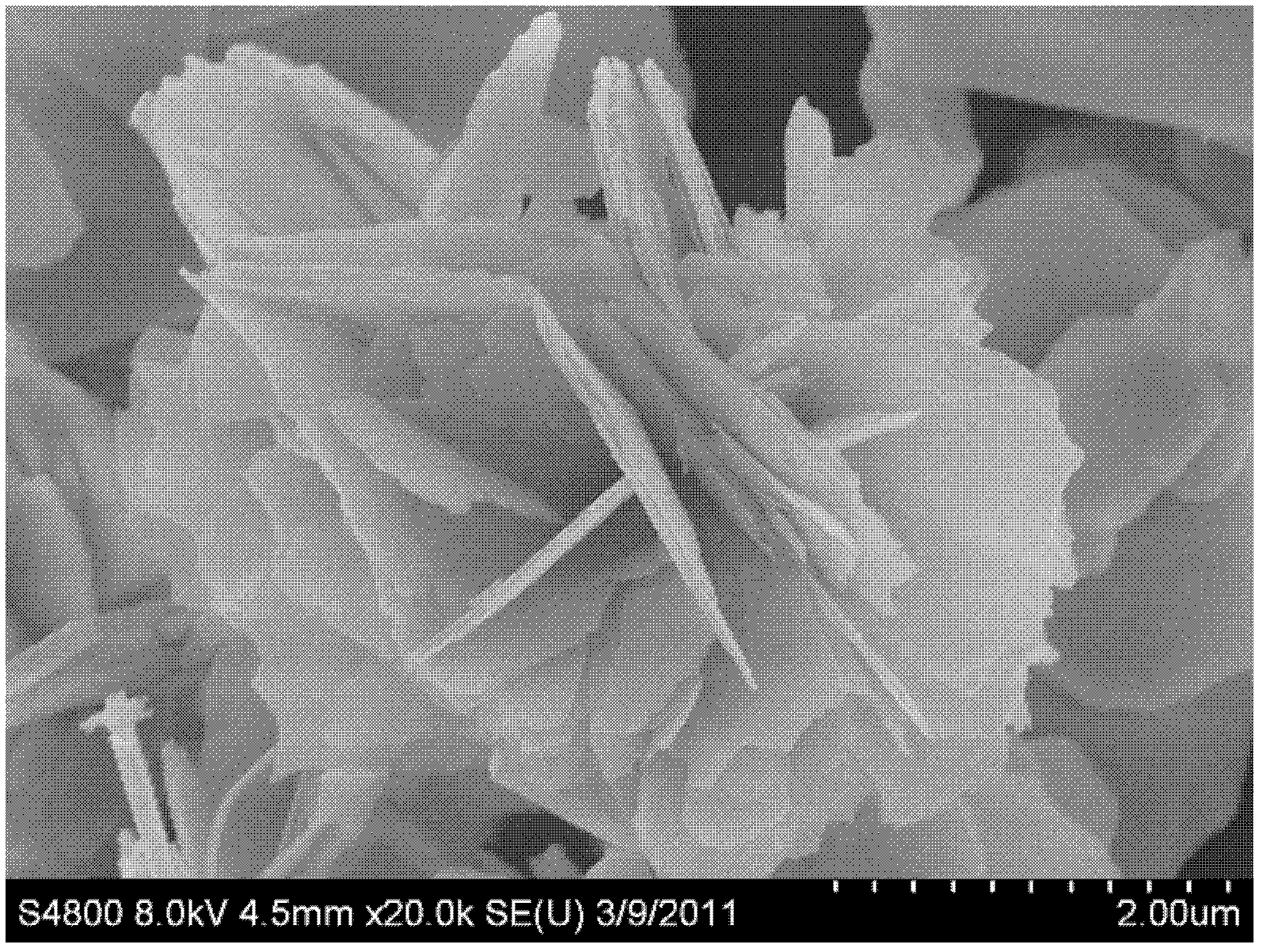
[0056] (2) 0.823g of sodium phosphate is uniformly dissolved in 50mL of deionized water to obtain a phosphate-containing soluble saline solution, and the phosphate-containing soluble saline solution is added dropwise to the solution obtained in step (1), and placed in a high-pressure reaction Reaction in the tank at 120°C for 24 hours, the reaction product was filtered through a suction filter and then filtered through filter paper, then washed with alcohol for 3 times and then washed with water for 3 times, and dried in vacuum at 60°C for 6 hours to obtain a white phosphate material. Its scanning electron microscope image is figure 1 As shown, it is a long rod-shaped Sr with a diameter of 50-100 nm and a length of 0.5-2 microns. 5 (P...
Embodiment 2
[0058] (1) Dissolve 2 g of strontium nitrate in 100 mL of deionized water, add 0.5 g of sodium dodecylbenzene sulfonate, stir thoroughly for 30 min to disperse it evenly, adjust pH=1 with 0.1 mol / L dilute nitric acid, and obtain solution.
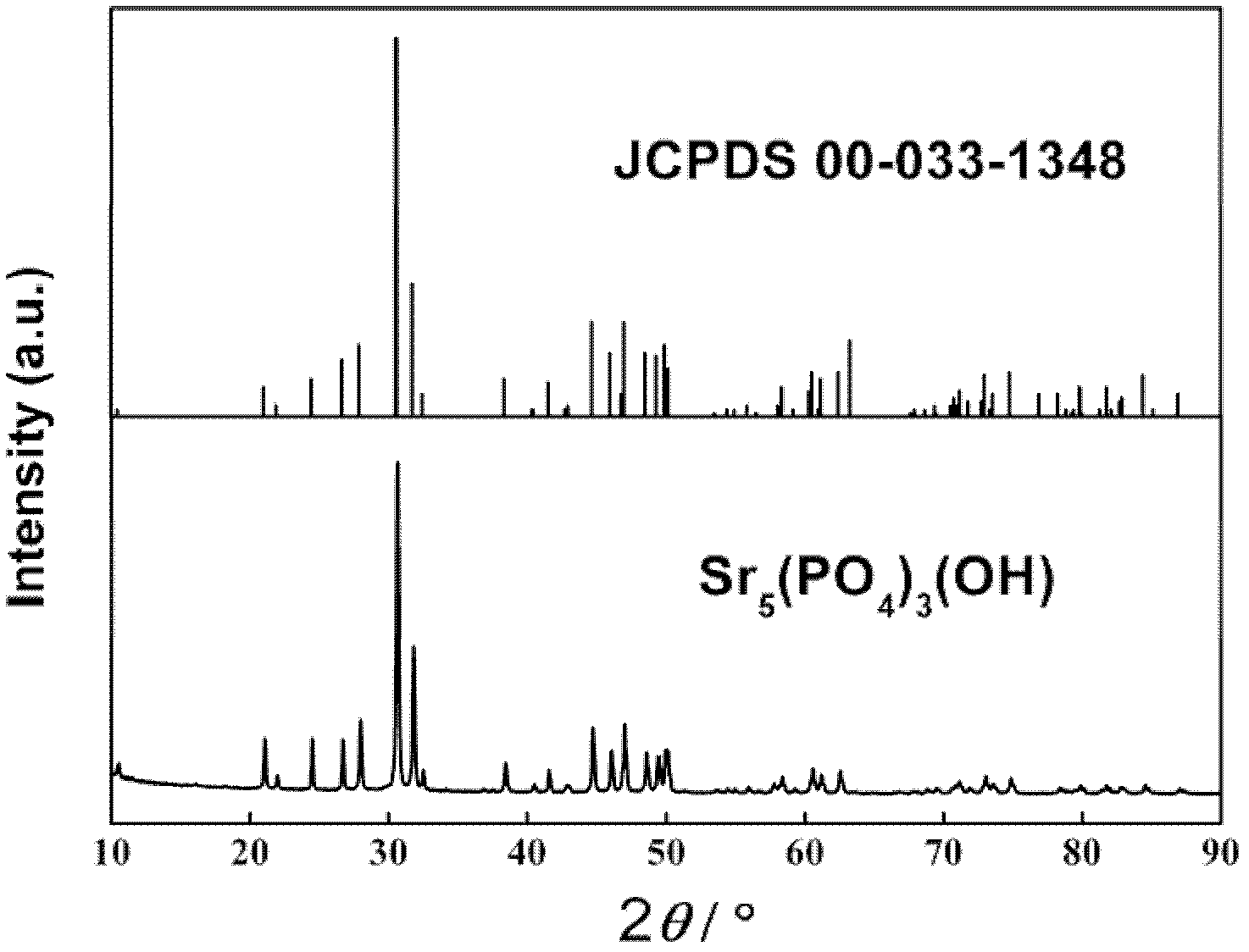
[0059] (2) Dissolve 2 g of sodium dihydrogen phosphate evenly in 50 mL of deionized water to obtain a phosphate-containing soluble saline solution, and add the phosphate-containing soluble saline solution dropwise to the solution obtained in step (1), at 20° C. React for 24 hours, the reaction product is filtered through a suction filter and then filtered through filter paper, then washed with alcohol for 3 times, then washed with water for 3 times, and dried in vacuum at 50°C for 18 hours to obtain a white phosphate material with a diameter of 20-70nm , short rod-shaped SrHPO with a length of 100-50 nm 4 , the X-ray diffraction spectrum of the phosphate material is shown as Figure 4 shown.
Embodiment 3
[0061] (1) Dissolve 2.61g of barium nitrate in 100mL of deionized water, add 1.5g of hexadecyltrimethylammonium bromide, stir thoroughly for 30min to disperse evenly, adjust the pH with 0.01mol / L dilute nitric acid = 5. Get a solution.
[0062] (2) Dissolve 10 g of sodium phosphate evenly in 100 mL of deionized water to obtain a phosphate-containing soluble saline solution, add the phosphate-containing soluble saline solution dropwise to the solution obtained in step (1), and place in a high-pressure reaction tank Reaction at 180°C for 8 hours, the reaction product was filtered by a suction filter, filtered through filter paper, washed with alcohol for 3 times, washed with water for 4 times, and dried in vacuum at 40°C for 24 hours to obtain a white phosphate material. Microscope picture as figure 2 As shown, it is flaky Ba with a thickness of 10-50nm and a diameter of 1-2 microns 3 (PO 4 ) 2 (abbreviation BP); The X-ray diffraction spectrogram of this phosphate material ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com