Method for preparing curvularin and indolizidine alkaloid and application
A technology of Curvus sp. and Curvus sp., which is applied in the field of new indolizidine alkaloids and their preparation, can solve the problem that the metabolites of special environment microorganisms do not attract enough attention, and achieves short cycle, simple process, Effects of strong acetylcholinesterase inhibitor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Curvularia IFB-Z10 of marine origin ( Curvularia sp . ) separation and purification
[0029] Dissect the white meager under aseptic conditions, disinfect the surface of the taken out meager intestines in 75% alcohol for 2 minutes, rinse with sterile water for 3 times, add a small amount of sterile water and grind them in a sterile mortar. Gradiently dilute the polishing solution with sterile water to 10 -1 , 10 -2 , 10 -3 , 10 -4 , respectively take 0.2 ml of each gradient dilution and spread it on Chase medium (sucrose 30 g, NaNO 3 3.0 g, NaCl 0.5 g, K 2 HPO 4 1.0 g, MgSO 4 ·7H 2 O 0.5 g, FeSO 4 ·7H 2 O 0.01 g, 1 L of distilled water), cultured in a 28°C incubator, and after the colonies grew out, the mycelium was picked and purified to obtain the intestinal fungus IFB-Z10 of white meager fish, which was tested by molecular biology and morphology identified as Curvularia IFB-Z10 ( Curvularia sp . ), is now deposited in the China Center for...
Embodiment 2
[0030] Embodiment 2: Curvularia IFB-Z10 of marine origin ( Curvularia sp.) liquid fermentation
[0031] Activation of marine-derived Curvularia IFB-Z10 ( Curvularia sp.), inoculate fresh bacterium blocks into 1000 ml Erlenmeyer flasks, each bottle contains 400 ml of modified Chapeauer medium, inoculate 5-6 bottles on a shaker, at 150 rpm, 28-30 ℃ Cultivate for 3 days as the seed solution, then inoculate the seed solution with 20 ml inoculum in a 1000 ml Erlenmeyer flask containing 400 ml of modified Chapeauer's medium, and ferment for 12 days at 150 rpm and 28-30 °C.
Embodiment 3
[0032] Embodiment 3: Extraction and separation of indolizidine alkaloids
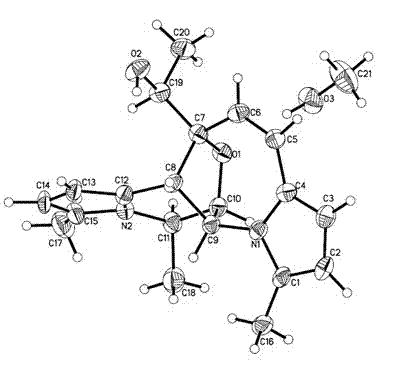
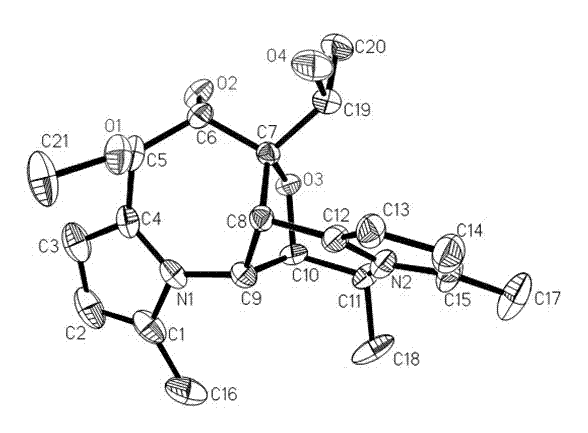
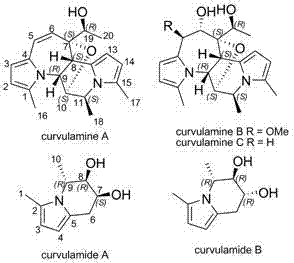
[0033] The fermented liquid obtained in Example 2 was filtered through gauze, the filtrate was extracted with ethyl acetate, concentrated and dried in vacuo to obtain black crude extract F1. Carry out silica gel column chromatography on extract F1, use 4 times the volume of chloroform successively, chloroform:methanol=100:1, chloroform:methanol=100:2, chloroform:methanol=100:4, carry out elution, concentrate 100:1 Segment obtains black extract F2; 100:4 segment obtains black extract F3; Then Sephadex LH-20 separates extract F2 to obtain curvulamine A, and merges other fractions to obtain brown extract F2-1; extract F2- 1 with high pressure liquid chromatography (column: Allsphere ODS 5 mu m (250×4.6 mm) column; mobile phase: methanol / water=80 / 20, flow rate: 2.0 mL / min) to separate curvulamine B (retention time t R =26.1 min); curvulamine C (retention time t R =22.3 min); for the extract F3, use hi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com