Coating membrane for micro pill tabletting and preparation method for coating membrane
A technology of pellet coating and coating materials, which is applied in the field of pharmaceutical preparations, can solve the problems of increased process difficulty, large dosage, and slow release of pellets, and achieve the effects of reducing side effects, reducing dosage, and improving fracture strain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Coating film prescription:
[0048]
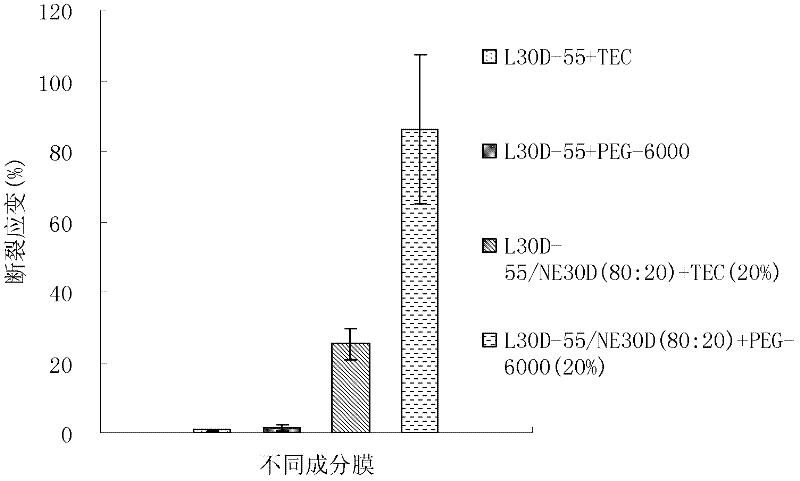
[0049] Adjust the pH of L30D-55 and NE30D to 5.0 with 1mol / L NaOH(aq) and 1mol / L HCl(aq) respectively, and slowly pour NE30D into L30D-55 on a magnetic stirrer. Weigh double-distilled water in a small beaker, add the weighed polyethylene glycol 6000 and propylene glycol into the double-distilled water, stir fully until dissolved, slowly pour the dissolved mixed solution into the mixed suspension of the polymer, and keep stirring at a low speed State 5h. The dosage of the macromolecule plasticizer for the coating film is 20% of the dry weight of the polymer.
[0050] Pass the above suspension through a 80-mesh sieve, pour it into a flat 20cm×10cm×3cm glass pool, spread it evenly with a smear bar, and dry it in a blast oven at 40°C for 5 hours. Measure the thickness of the dried film with a vernier caliper, take a film with a thickness of 180 μm to 220 μm, and cut it into a dumbbell shape. According to the standard DIN ISO 527-3...
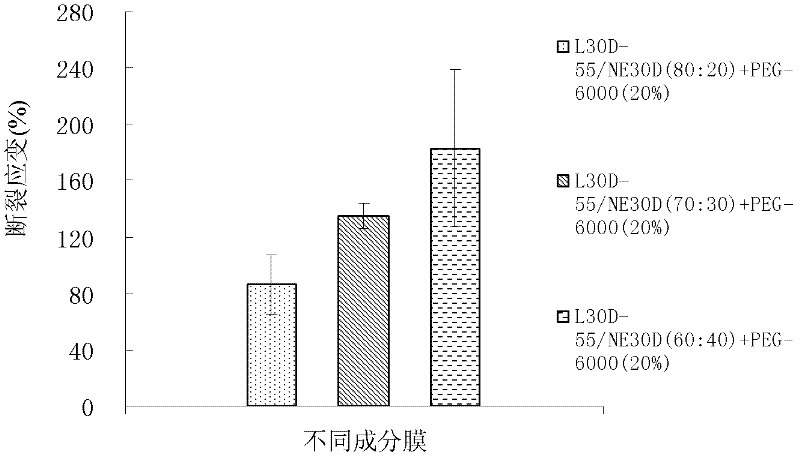
Embodiment 2
[0061] Coating film prescription:
[0062]
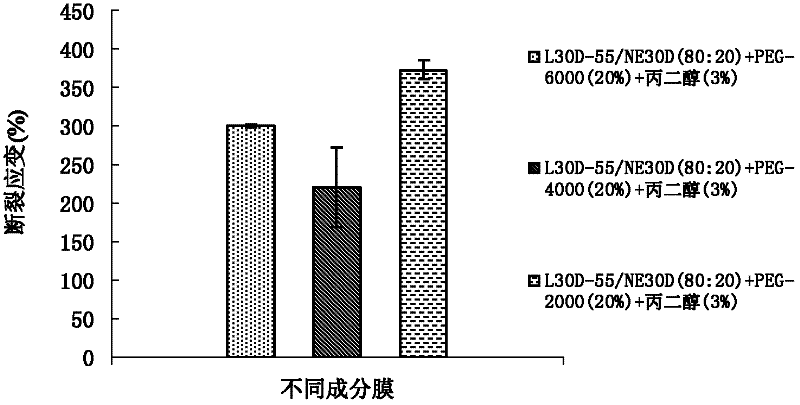
[0063]The preparation method of coating liquid is the same as embodiment 1. The amount of the macromolecule plasticizer polyethylene glycol 6000 in the coating film prescription is 20% of the dry weight of the polymer, and the amount of the small molecule plasticizer propylene glycol is 5% of the dry weight of the polymer. Compared with the embodiment 1 that the addition of small molecular plasticizer propylene glycol is 3%, the results are shown in Table 2.
[0064] Table 2
[0065]
[0066] As can be seen from the results in Table 2, in L30D-55 / NE 30D (80:20), after adding polyethylene glycol 6000 of 20% polymer dry weight, along with the addition of small molecule plasticizer propylene glycol from 3% To 5%, the breaking strain of the coating film increases from about 290 to about 400 thereupon. If the amount of propylene glycol continues to increase, it can be found in practical application that the viscosity of the coati...
Embodiment 3
[0068] Diclofenac sodium (DS) enteric-coated pellets
[0069]
[0070] DS drug-loaded pellets are prepared by adding drug to a blank pellet core, adding 1.5% (w / w) HPMC E5 solution to DS to prepare a 10% (w / w) DS drug solution, spraying drug on the bottom of the fluidized bed, 100% weight gain on medication. The preparation method of the enteric layer is the same as that in Example 1, using the fluidized bed bottom spray coating method, based on the actual coating weight, the enteric coating is wrapped on the basis of the DS drug-loaded pellets, and the macromolecular plasticizer polyethylene glycol 6000 The dosage is 20% of the dry weight of the polymer, and the weight gain of the enteric layer is 10% of the weight of the DS drug-loaded pellets. After the coating is finished, spread it flat on a tray and place it in a 38°C oven for aging for 12 hours.
[0071] DS enteric-coated pellets and tablets
[0072]
[0073]
[0074] The DS enteric-coated pellets prepared a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fracture strain | aaaaa | aaaaa |
| fracture strain | aaaaa | aaaaa |
| fracture strain | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com