Method for producing lithium cobaltite by preparing hydroxyl trivalent cobalt oxide through wet chemical reaction
A hydroxyl trivalent and chemical reaction technology, applied in chemical instruments and methods, inorganic chemistry, cobalt compounds, etc., can solve the problems of slow oxygen diffusion, small profit margins, and poor reaction kinetic conditions, and achieve uniform product quality, The effect of good product quality and fast sintering reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Main raw materials: cobalt sulfate and sodium hydroxide are industrial-grade products, 20% ammonia water is an industrial-grade product, lithium carbonate is a battery-grade product, and sodium hypochlorite solution is an analytical reagent. These main raw materials are commercially available.
[0044] 1. Solution preparation: 0.75M cobalt sulfate solution, 5.50M sodium hydroxide solution.
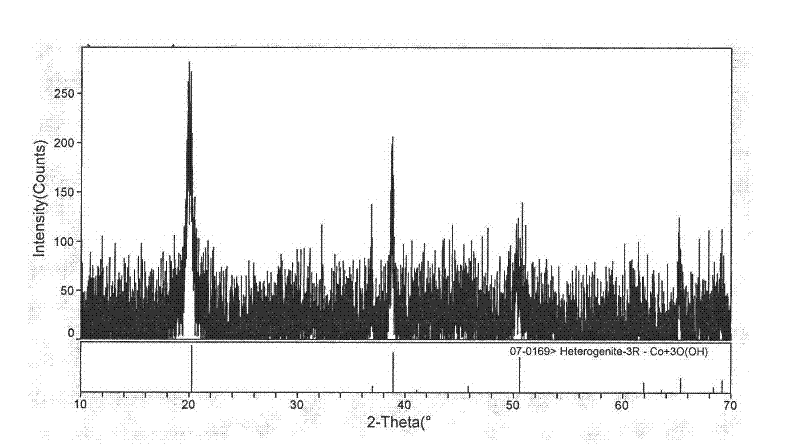
[0045] 2. Preparation of cobalt oxyhydroxide precursor
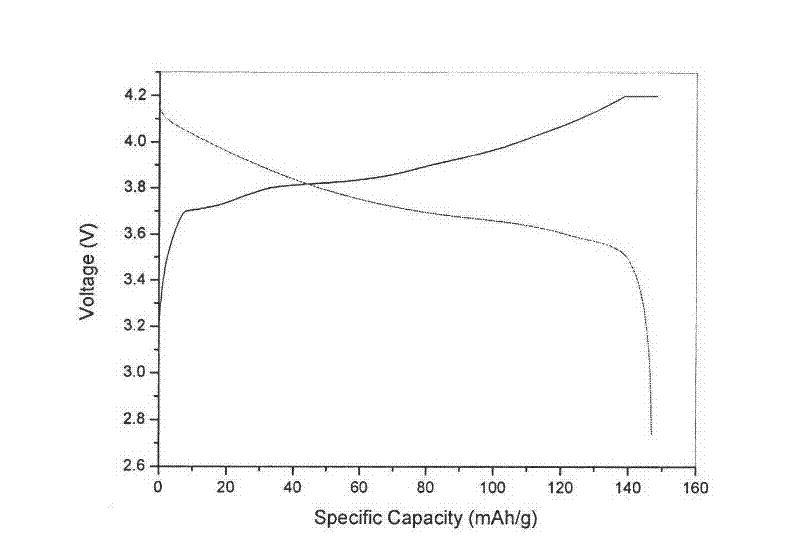
[0046] Add 0.70M cobalt sulfate solution into the reactor, heat to 60°C, add 5.26M sodium hydroxide solution into the reactor according to stoichiometry, the feeding time is 55 minutes, the alkali excess coefficient is 1.10, and the mass concentration is 20 % ammonia solution is used as a complexing agent, the pH value is adjusted to 9-14, and the stirring speed of the reactor is controlled to be 350 rpm. After the lye was added, the reaction was stirred at a constant temperature of 60° C. for 3 hours. Obtain a very viscous ...
Embodiment 2
[0052] Main raw material: same as embodiment 1.
[0053] 1. Solution preparation: 1.00M cobalt chloride solution, 5.26M sodium hydroxide solution.
[0054] 2. Preparation of cobalt oxyhydroxide precursor
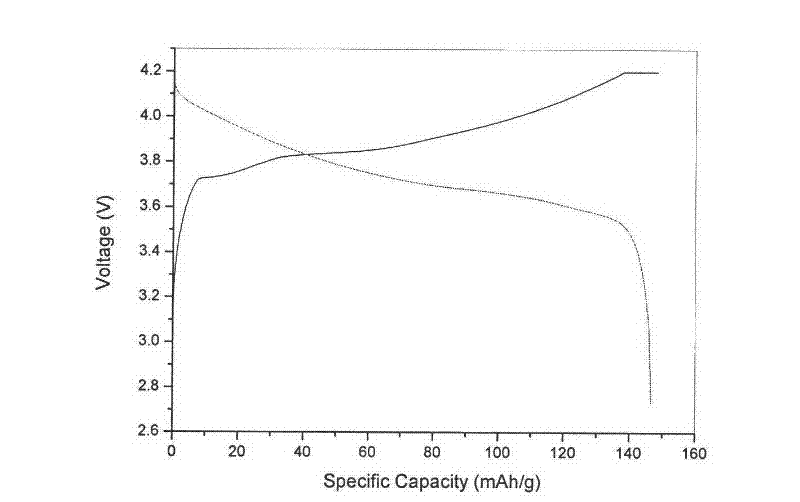
[0055] Add 1.00M cobalt chloride solution in the reactor, heat to 70°C, slowly add 5.26M sodium hydroxide solution into the reactor according to stoichiometry, the alkali excess coefficient is 1.05, and add a mass concentration of 20% ammonia solution at the same time as complexing agent, adjust the pH value to 9-14, and control the stirring speed of the reactor to 300 rpm. After adding the lye, stir and react at 300 rpm at a constant temperature of 60°C for 3.5 hours. Obtain very viscous cobaltous hydroxide slurry, then add 1.0ml of analytically pure sodium hypochlorite solution per gram of cobalt in the reaction tank, stir at 300 rpm for 4 hours, and the oxidation temperature is 60°C, so that the cobaltous hydroxide material The slurry is fully oxidized to obtain a coba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com