Synthesis method of hydroxytyrosol
A technology for the synthesis of hydroxytyrosol, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of high cost, unsuitability for industrial production, poor stability of raw materials, etc., and achieve low cost and good production conditions And the effect of low equipment requirements and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
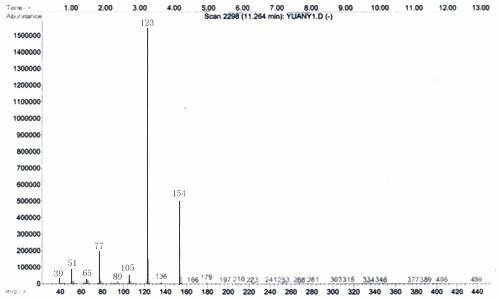
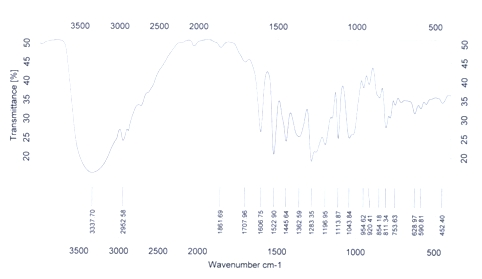
[0017] Under the protection of argon, 0.55g (23.9mmol) of sodium chips was added to the pre-dried three-necked flask, and 1.0g (4.7mmol) of 3,4-dimethoxyphenylacetic acid methyl ester was dissolved in 20mL of anhydrous The ethanol solution was slowly dropped into the three-necked flask, and after the reaction was stable, it was heated and refluxed for 1 hour. After cooling, the product solution was acidified with hydrochloric acid, and insoluble substances were precipitated, which were removed by suction filtration under reduced pressure. ×10mL ) extraction and column separation (chloroform) to obtain hydroxytyrosol. The product is light yellow oily liquid, and the yield is 50%. 1 H NMR (400MHz, CDCl 3 ): δ= 6.60 (d, 1H), 6.58 (d, 1H), 6.43 (d, 1H), 3.47 (t, 2H), 2.52 (t, 2H); Ms m / z: 154(M + ); IR (KBr) υ: 3337, 2953, 1708, 1607, 1523, 1446, 1363, 1283, 1044, 955, 920, 855, 811, 753.cm -1 . ( figure 2 )
Embodiment 2
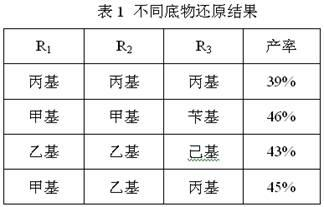
[0019] Under the protection of argon, add 0.27g (12mmol) of sodium chips into the pre-dried three-necked flask, and slowly dissolve 1.0g (4.2mmol) of 3,4-diethoxyphenylacetic acid methyl ester in 30mL of methanol solution under stirring Drop it into a three-neck flask, and after the reaction is stable, heat and reflux for 1 hour. After cooling, the product solution is acidified with hydrochloric acid, and insoluble substances are precipitated, which are removed by suction filtration under reduced pressure. Hydroxytyrosol can be obtained by extraction and column separation (chloroform). The product is a pale yellow oily liquid with a yield of 47%, and the NMR, mass spectrometry, and infrared data are the same as in Example 1.
Embodiment 3
[0021] Under the protection of argon, 0.55g (23.9mmol) of sodium chips was added to the pre-dried three-necked flask, and 1.0g (4.0mmol) of ethyl 3,4-diethoxyphenylacetate in 20mL of isopropyl The alcohol solution was slowly dropped into the three-necked flask, and after the reaction was stable, it was heated and refluxed for 1 hour. After cooling, the product solution was acidified with hydrochloric acid, and insoluble substances were precipitated, which were removed by suction filtration under reduced pressure. Then the clear liquid was concentrated and washed with chloroform (3 ×10mL) extraction and column separation (chloroform) to obtain hydroxytyrosol. The product is a light yellow oily liquid with a yield of 43%, and the NMR, mass spectrometry, and infrared data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com