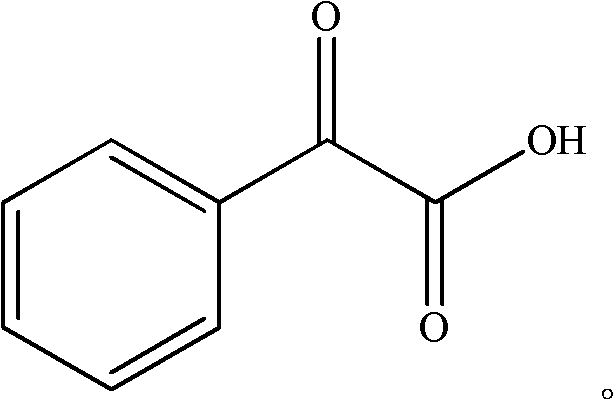
High selectivity synthesis method of benzoyl formic acid
A technology of benzoylformic acid and a synthesis method, which is applied in the field of highly selective synthesis of benzoylformic acid, can solve problems such as unfavorable large-scale industrial production sustainable development, backward benzoylformic acid method, serious environmental pollution, etc. The effect of reducing wastewater discharge, improving product yield and purity, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
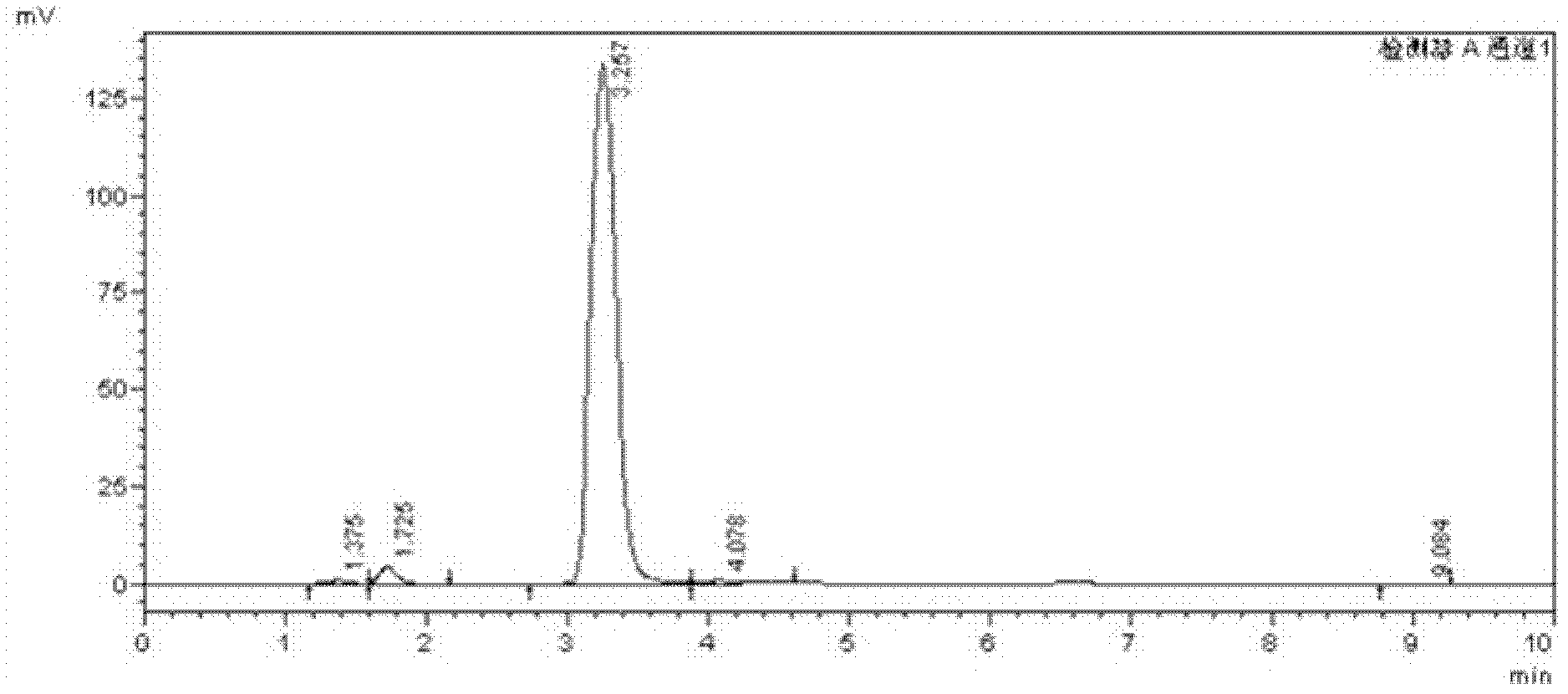
Examples
Embodiment 1
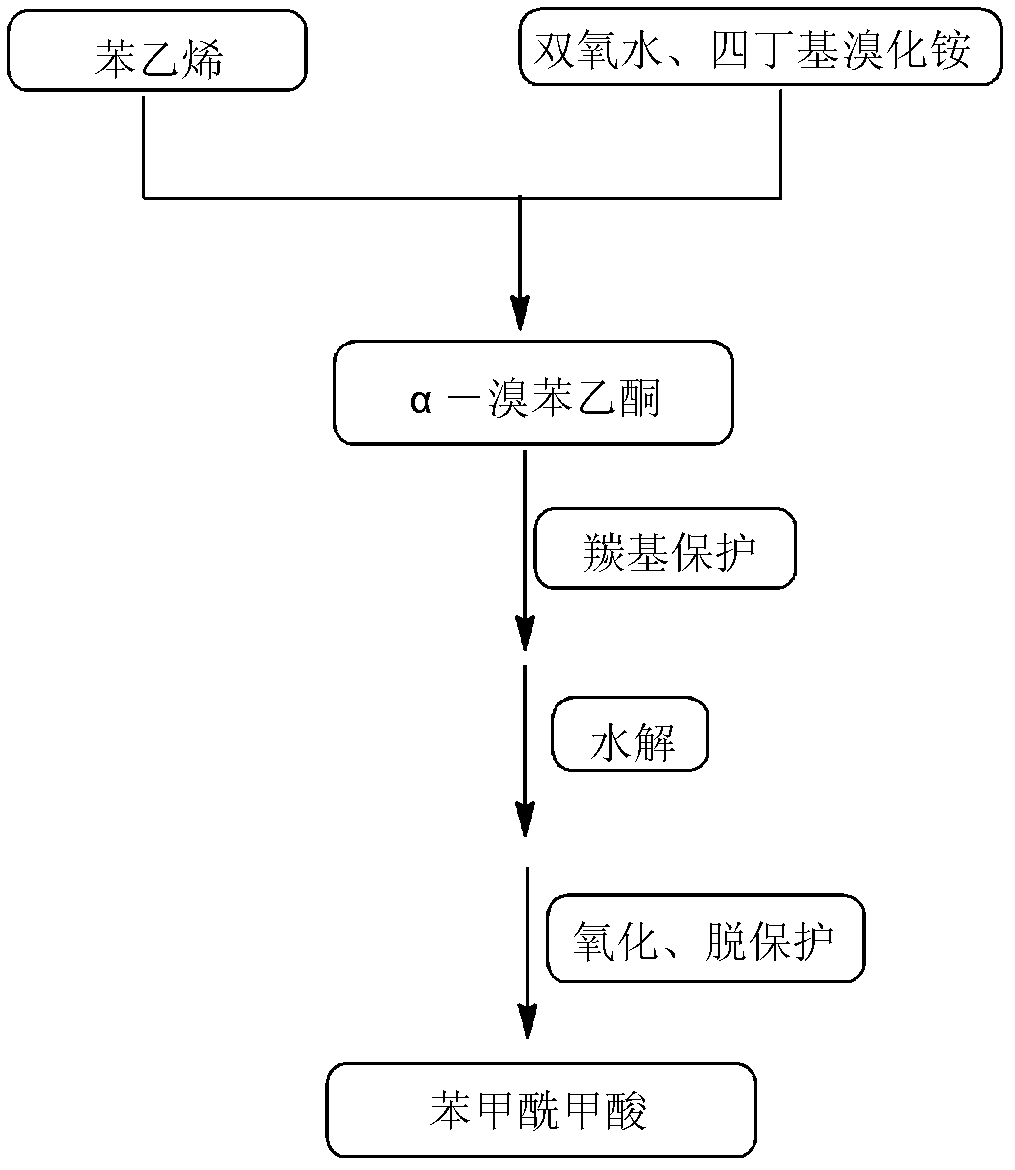
[0046] 1. At 10-25°C, add 150mL of water, 10.40g of styrene and 35.42g of tetrabutylammonium bromide into a 500mL three-necked flask, slowly add 39.67g of hydrogen peroxide solution with a mass fraction of 30% under stirring, and control the drop Adding time is 1-1.5h. After the dropwise addition, slowly raise the temperature to 60-70°C for 10-13h. After the reaction, cool down to room temperature. Separate the organic phase and the water phase by standing still, and extract the organic phase with ethyl acetate for 2 -3 times, the extracts were combined, the solvent was distilled off under reduced pressure at 0.3-0.5MPa, 40-45°C, and then recrystallized with dichloromethane to obtain 18.90g of α-bromoacetophenone;
[0047] 2. Add the generated α-bromoacetophenone into a reactor containing 14.72g of ethylene glycol and 0.04g of p-toluenesulfonic acid, and react at 80-90°C for 30-40h. Extract the liquid with ethyl acetate for 2-3 times, combine the extracts, distill the extracts...
Embodiment 2
[0052] 1. At 10-25°C, add 150mL of water, 10.40g of styrene and 41.86g of tetrabutylammonium bromide into a 500mL three-necked flask, slowly add 39.67g of hydrogen peroxide solution with a mass fraction of 30% under stirring, and control the drop Adding time is 1-1.5h. After the dropwise addition, slowly raise the temperature to 60-70°C for 10-13h. After the reaction, cool down to room temperature. Separate the organic phase and the water phase by standing still, and extract the organic phase with ethyl acetate for 2 -3 times, the extracts were combined, the solvent was distilled off under reduced pressure at 0.3-0.5MPa, 40-45°C, and then recrystallized with dichloromethane to obtain 19.10g of α-bromoacetophenone;
[0053] 2. Add the generated α-bromoacetophenone into a reactor containing 16.07g of ethylene glycol and 0.04g of p-toluenesulfonic acid, and react at 80-90°C for 30-40h. Extract the liquid with ethyl acetate for 2-3 times, combine the extracts, distill the extracts...
Embodiment 3
[0058] 1. At 10-25°C, add 150mL of water, 10.40g of styrene and 41.86g of tetrabutylammonium bromide into a 500mL three-necked flask, slowly add 45.33g of hydrogen peroxide solution with a mass fraction of 30% under stirring, and control the drop Adding time is 1-1.5h. After the dropwise addition, slowly raise the temperature to 60-70°C for 10-13h. After the reaction, cool down to room temperature. Separate the organic phase and the water phase by standing still, and extract the organic phase with ethyl acetate for 2 -3 times, the extracts were combined, the solvent was distilled off under reduced pressure at 0.3-0.5MPa, 40-45°C, and then recrystallized with dichloromethane to obtain 19.36g of α-bromoacetophenone;
[0059] 2. Add the generated α-bromoacetophenone into a reactor containing 18.10g of ethylene glycol and 0.04g of p-toluenesulfonic acid, and react at 80-90°C for 30-40h. Extract the liquid with ethyl acetate for 2-3 times, combine the extracts, distill the extracts...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com