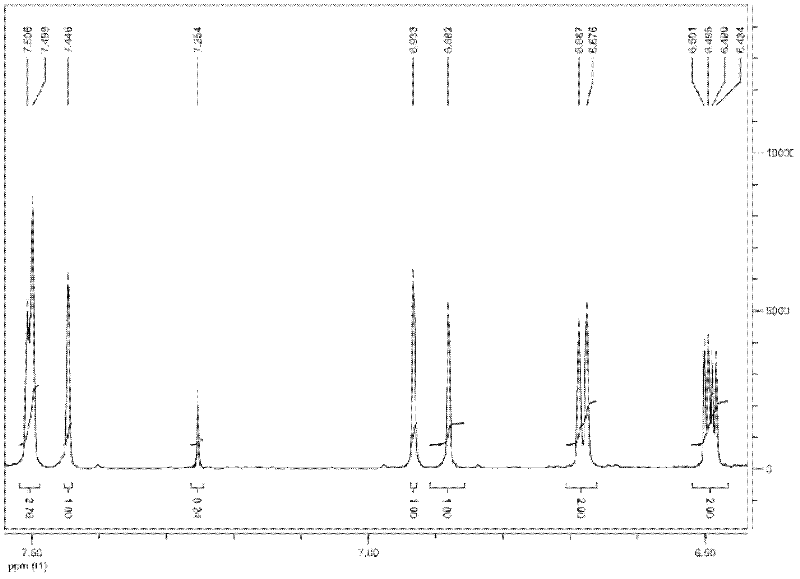
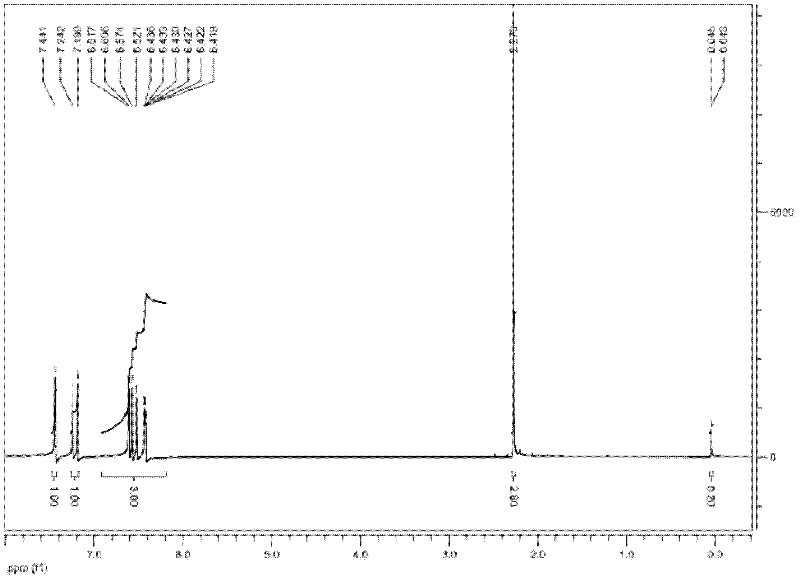
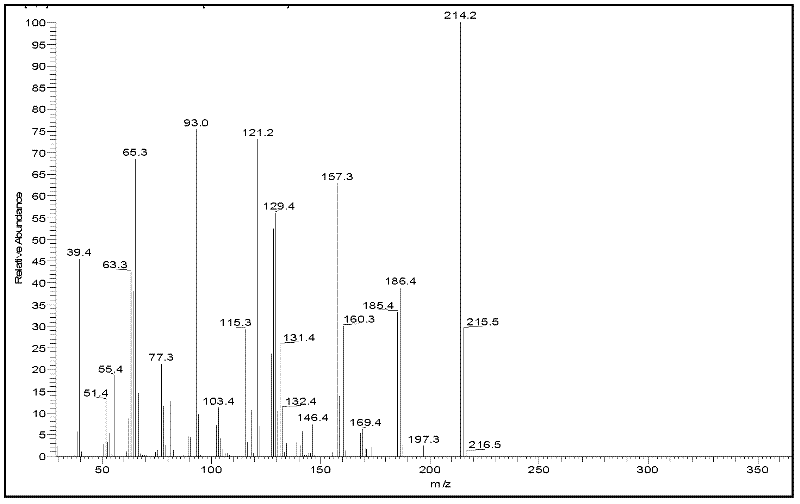
Method for preparing furfurylideneacetone and di-furfurylideneacetone from furfural
A technology of difurfurylidene acetone and furfurylidene acetone, which is applied in the chemical field, can solve the problems of non-toxicity, strong corrosion and pollution, and achieve the effect of simple separation and purification process, high overall yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 4.8g furfural (0.05mol), 2.9g acetone (0.05mol) and 1.06g 20%-MgO / NaY catalyst are mixed with 95.4g (105ml) volumetric concentration in aqueous ethanol solution of 50%, furfural concentration is 4.6wt%, put Into a three-necked flask reactor equipped with a reflux column, heated to 100°C in an oil bath, and reacted for 8 hours. After the reaction is finished, cool for 30 minutes, vacuum filter, separate the catalyst, and collect the reaction solution. The collected reaction solution was transferred to a refrigerator set at -3°C for 8 hours, then suction filtered, and the crystals on the filter paper were collected, air-dried at room temperature for a period of time, and weighed to obtain 2.45 g of difurfurylideneacetone.
[0040] Transfer the remaining reaction liquid to a rotary hair dryer, fully recover ethanol at 60°C, and remove the ethanol solution in the recovery bottle when the reaction liquid becomes yellow and turbid. Then raise the temperature to 120°C, contin...
Embodiment 2
[0042] 4.8g furfural (0.05mol), 2.9g acetone (0.05mol) and 1.06g 20%-MgO / NaY catalyst and 95.4g (105ml) volume concentration are 40% ethanol aqueous solution mixing, furfural concentration is 4.6wt%, in Reaction at 100°C for 8h. The remaining steps were the same as in Example 1, 2.54 g of difurfurylidene acetone and 1.68 g of difurfurylidene acetone were collected, and the total yield was 54.8%.
Embodiment 3
[0044] Get 4.8g furfural (0.05mol), 5.8g acetone (0.1mol) and 1.06g 50%-MgO / NaY catalyst and 95.4g (105ml) volume concentration is the ethanol aqueous solution mixing of 50%, furfural concentration is 4.5wt%, in The reaction was carried out at 100° C. for 8 hours, and the remaining steps were the same as in Example 1. 2.35 g of difurfurylidene acetone and 1.78 g of difurfurylidene acetone were collected, and the total yield was 38.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com