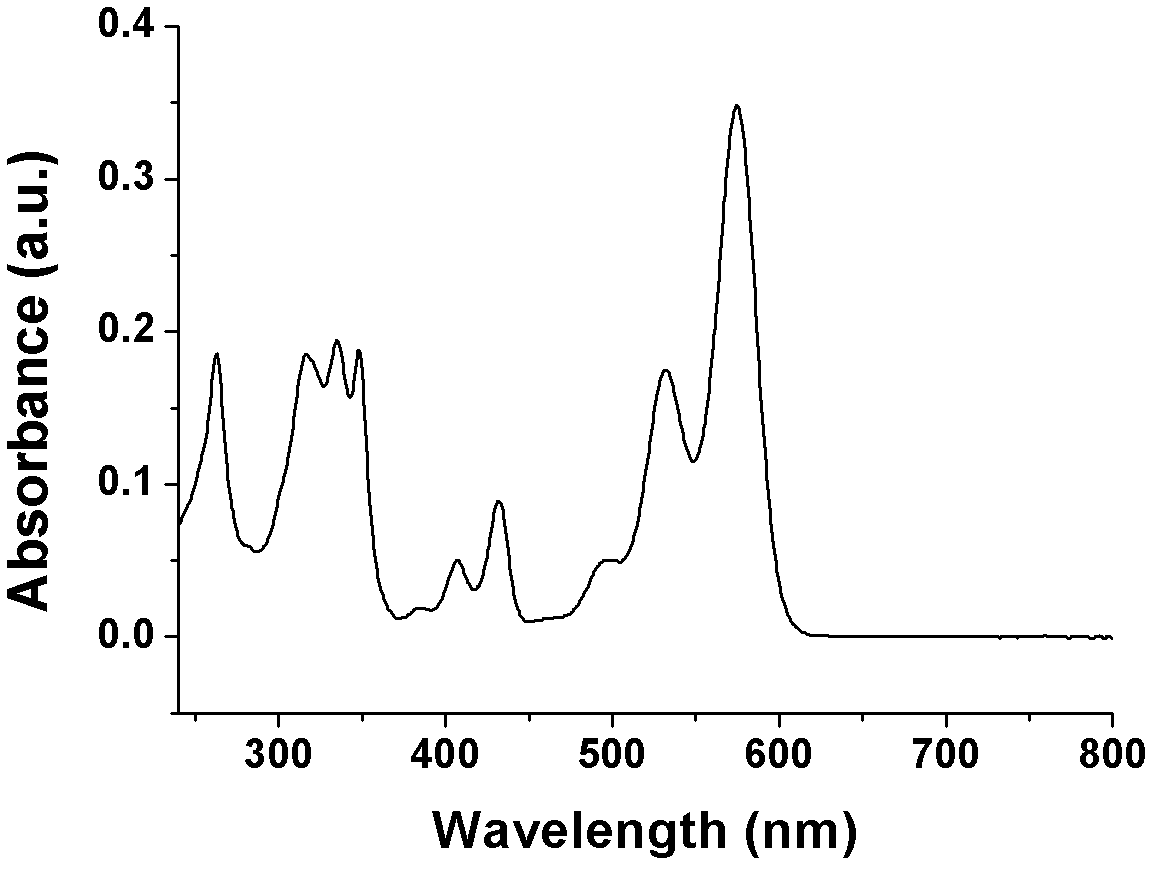
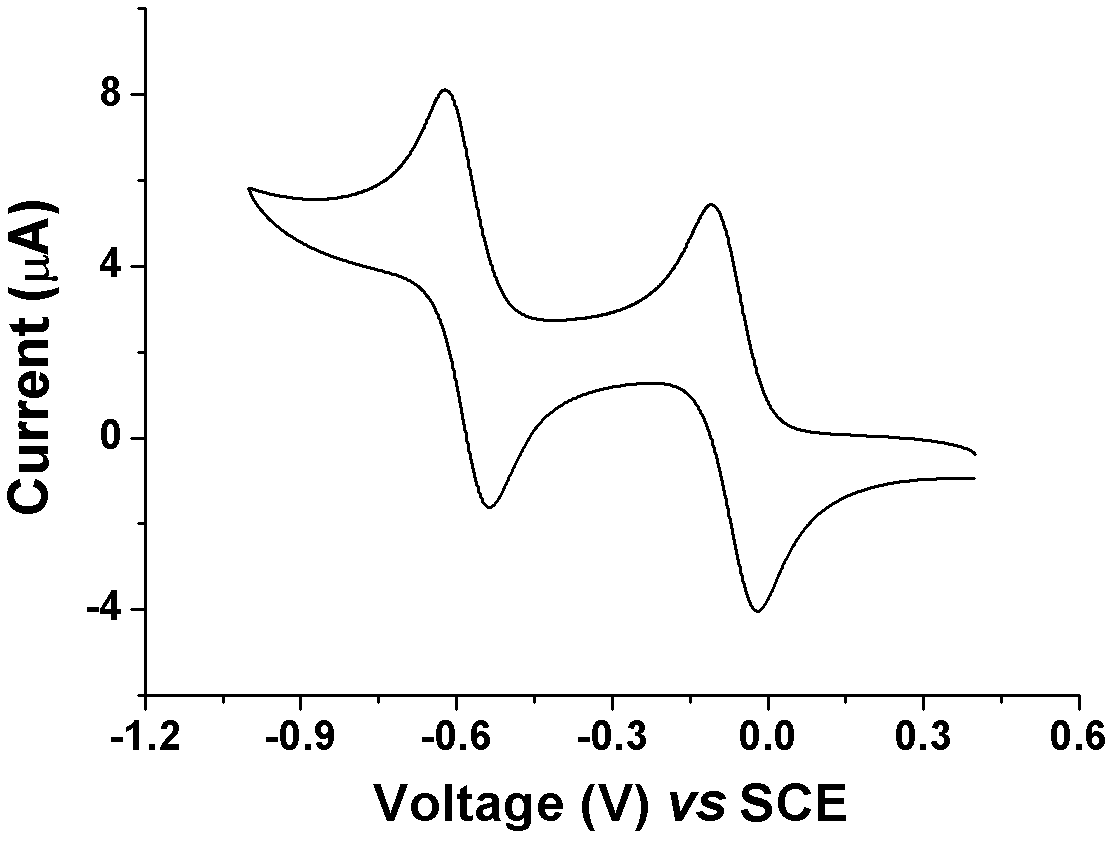
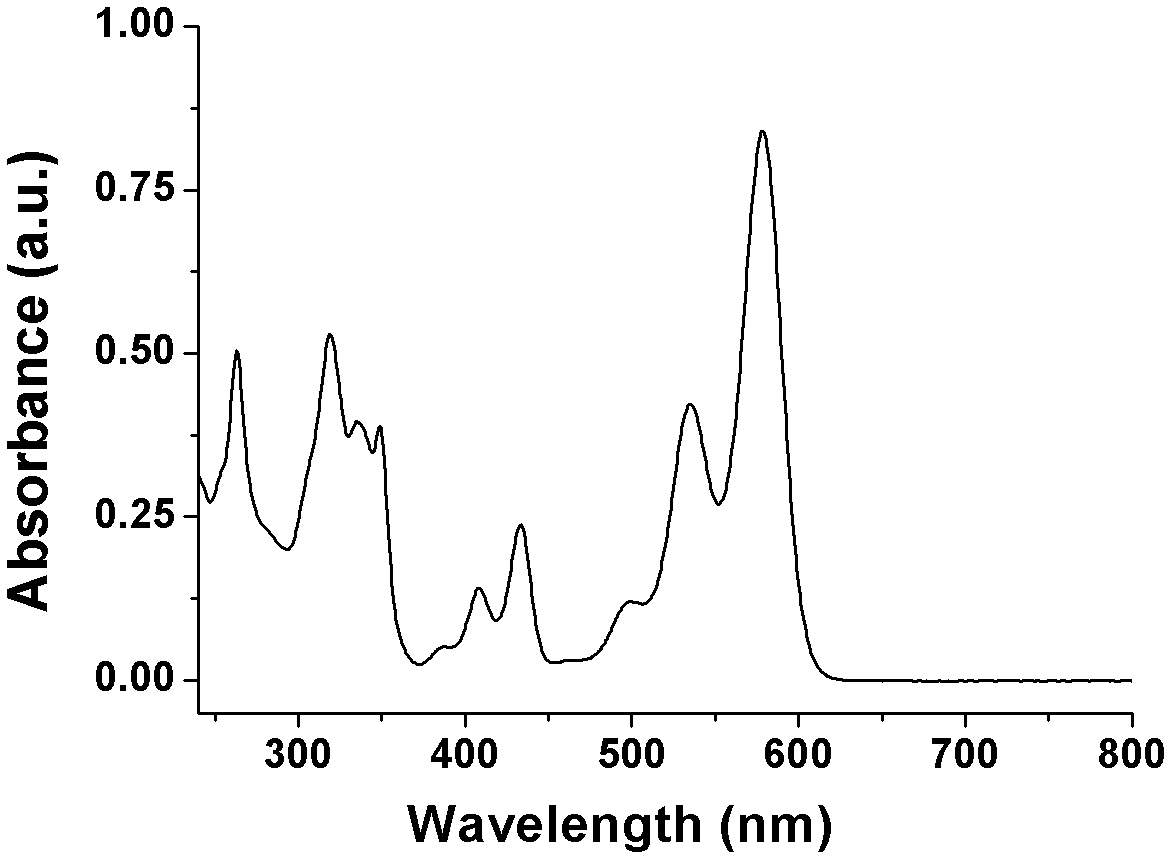
Heterocyclic-sulfur fused naphthalene diimide compounds, preparation method and application thereof
A naphthalene diimide and compound technology, which is applied in the field of bis[2-malononitrile] fused naphthalene diimide compounds, can solve the problem of single types of n-type organic semiconductor materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: N, N'-bis(2,2-bis(octyloxy)-1-ethyl)-[2,3-d:6,7-d']-bis[2-(1, Synthesis of 3-dithiacyclopentene-2-ylidene)-2-propanedicyano]-naphthalene-1,4,5,8-tetracarboxylic diimide (1).
[0110]
[0111] Under nitrogen protection, 2,3,6,7-tetrabromonaphthalene tetracarboxylic dianhydride (TBNDA) (100mg, 0.17mmol) and 1,1-dicyanoethylene-2,2-dithiolate sodium (96mg , 0.52mmol) was added into 15mL DMF and heated to 50°C. After stirring for 1 h, 2,2-bis(octyloxy)ethylamine (207 mg, 0.68 mmol) was added, and the temperature was maintained for 7 h. Cool to room temperature, pour the reaction solution into 100 mL of saturated ammonium chloride solution, filter, wash the filter residue with water, dry in vacuo, use dichloromethane / petroleum ether (2:1) as eluent, and use a silica gel chromatography column to analyze the crude The product was separated and purified to obtain compound 1 as a brown-red solid with a yield of 42%. Mass spectrum: MS (EI) m / z 1110.8 (M + ); H N...
Embodiment 2
[0112] Example 2: N, N'-bis(4-tert-butylphenyl)-[2,3-d:6,7-d']-bis[2-(1,3-dithiecyclopentene- Synthesis of 2-ylidene)-2-propanedicyano]-naphthalene-1,4,5,8-tetracarboxylic diimide (2).
[0113]
[0114] Using 4-tert-butylaniline instead of 2,2-bis(octyloxy)-ethyl-1-amine, the synthesis method was the same as in Example 1 to obtain compound 2 as a dark red solid with a yield of 55%. Mass Spectrum: MS (MALDI-TOF) m / z 807.1 (M + ); H NMR spectrum: 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 1.415 (s, 9H, -CH 3 ), 7.291-7.318 (d, 2H, H-Ph), 7.666-7.694 (d, 2H, H-Ph); elemental analysis calculated value (Anal. Calcd. For) C 42 h 26 N 6 o 4 S 4 : C, 62.51; H, 3.25; N, 10.41; Found: C, 62.49; H, 3.27; N, 10.54.
Embodiment 3
[0115] Example 3: N, N'-bis(3,4,5-tri(dodecyloxy)phenyl)-[2,3-d:6,7-d']-bis[2-(1 , Synthesis of 3-dithiacyclopentene-2-ylidene)-2-propanedicyano]-naphthalene-1,4,5,8-tetracarboxylic diimide (3).
[0116]
[0117] Replace 2,2-bis(octyloxy)-ethyl-1-amine with 3,4,5-tris(dodecyloxy)aniline, and the synthesis method is the same as in Example 1 to obtain 128 mg of blue-purple solid 3, producing rate 42%. Mass Spectrum: MS (MALDI-TOF) m / z 1799.3 (M + ); H NMR spectrum:1 H-NMR (300MHz, CDCl 3 )δ (ppm): 0.851-0.895 (m, 9H, -CH 3 ), 1.256-1.276 (m, 53H, -CH 2 -), 1.442-1.482(m, 6H), 1.775-1.844(m, 6H), 3.944-4.072(t, 6H, -CH 2 -O), 6.501 (s, 2H, H-Ph); C NMR spectrum: 13 C-NMR (100Hz, CDCl 3 ):δ14.104,14.115,22.653,22.680,22.701,22.719,26.096,26.132,29.310,29.360,29.401,29.416,29.638,29.656,29.705,29.714,29.769,29.779,29.787,30.406,31.918,31.925,31.948, 32.770, 69.367, 71.772, 73.738, 106.102, 111.309, 118.060, 125.450, 127.295, 139.539, 145.858, 154.182, 161.941, 181.278 (C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com