Preparation method of lithium ion battery anode material with high multiplying power
A technology for lithium-ion batteries and cathode materials, applied in battery electrodes, circuits, electrical components, etc., can solve problems that are not conducive to large-scale industrial production, complex and changeable processes, and excessive energy consumption, and achieve considerable reversible capacity, reversible Good controllability and stable cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
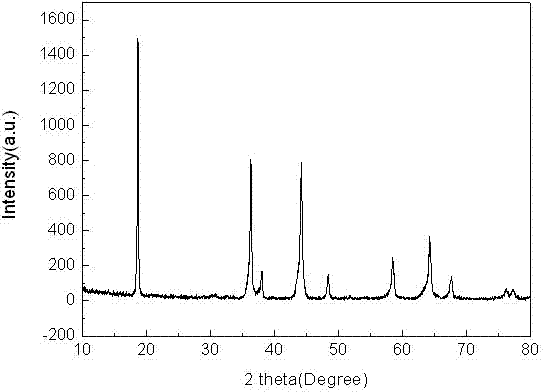
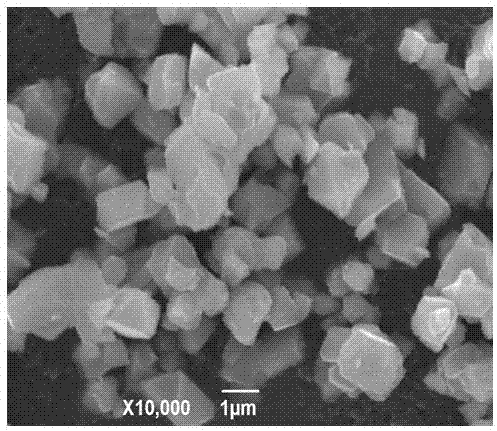
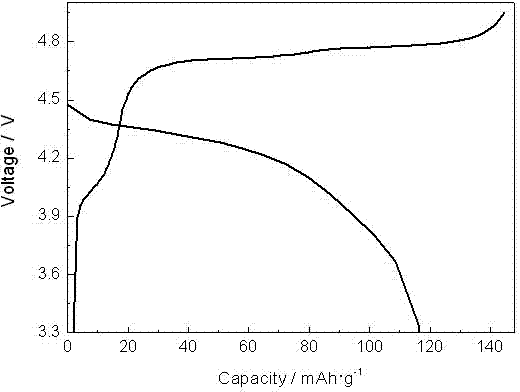
[0019] Example 1: Mix 0.205 mol of lithium hydroxide, 0.285 mol of manganese dioxide, 0.105 mol of nickelous oxide and 0.005 mol of niobium pentoxide, and then put them into a ball mill for ball milling for 8 h to make them evenly mixed, and then the final mixture Put it in a muffle furnace, raise it from room temperature to 850 °C at a rate of 5 °C / min, then react at 850 °C for 24 h, and then naturally cool to room temperature to obtain LiMn 1.425 Ni 0.525 Nb 0.05 o 4 . X-ray powder diffraction analysis indicated that the resulting LiMn 1.425 Ni 0.525 Nb 0.05 o 4 It is a pure phase, without any other impurity phase, and has high crystallinity. According to scanning electron microscope analysis, the particle size of the obtained product is uniform, and the particle size is 1-2 μm. The resulting product was used as an electrode material, and was assembled into an experimental button-type lithium-ion battery in a glove box filled with argon gas, with 0.1 C The charge cy...
Embodiment 2
[0020] Example 2: Mix 0.22 mol lithium nitrate, 0.285 mol manganese acetate, 0.105 mol nickel nitrate and 0.005 mol niobium pentoxide, and then put them into a ball mill for 5 h to make them evenly mixed, and then put the final mixture into a horse In a Furnace, rise from room temperature to 850 °C at a rate of 8 °C / min, react at 850 °C for 24 h, and then naturally cool to room temperature to obtain LiMn 1.425 Ni 0.525 Nb 0.05 o 4 . X-ray powder diffraction analysis indicated that the resulting LiMn 1.425 Ni 0.525 Nb 0.05 o 4 No impurities. According to scanning electron microscope analysis, the particle size of the obtained product is uniform and consistent, and the particle size is 3-4 μm. The resulting product was used as an electrode material, and was assembled into an experimental button-type lithium-ion battery in an argon-filled glove box at 0.1 C The charge cycle is performed at a rate of 3.3-4.95 V, and then the discharge cycle is performed at a rate of 3C, L...
Embodiment 3
[0021] Example 3: Mix 0.21 mol of lithium acetate, 0.285 mol of manganese nitrate, 0.105 mol of nickel acetate, and 0.01 mol of niobium hydroxide, and then put them into a ball mill for 10 h to make them evenly mixed, and then put the final mixture into a muffle In the furnace, rise from room temperature to 950 °C at a rate of 10 °C / min, react at 950 °C for 15 h, and then naturally cool to room temperature, that is, LiMn 1.425 Ni 0.525 Nb 0.05 o 4. X-ray powder diffraction analysis indicated that the resulting LiMn 1.425 Ni 0.525 Nb 0.05 o 4 No impurities. According to scanning electron microscope analysis, the particle size of the obtained product is uniform, and the particle size is 2-5 μm. The resulting product was used as an electrode material, and was assembled into an experimental button-type lithium-ion battery in a glove box filled with argon gas, with 0.1 C The charge cycle is performed at a rate of 3.3-4.95 V, and then the discharge cycle is performed at a r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com