Tetrathiafulvalene derivative, and organic film and organic transistor using the same
A technology of tetrathiafulvalene and its derivatives, which can be used in the field of raw materials for charge transport materials and organic electronic devices, and can solve problems such as mobility reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104]
[0105] The synthetic route of compound (3) is as follows.
[0106]
[0107] Synthesis process
[0108]
[0109] Methanol (100 mL) in which lithium chloride (1.00 g) had been dissolved was added dropwise to sodium borohydride (10.22 g), and then THF (50 mL) was added thereto. The resulting mixture was cooled to -10°C, and then 1,3-dithiol-2-thione-4,5-dimethyldicarboxylic acid dissolved in THF (100 mL) was added dropwise to the cooled mixture Esters: 1-1 (10.0 g). After the dropwise addition was complete, the resulting mixture was stirred in an ice bath for 3 hours. After the reaction was completed, the reaction solution was poured into ice water (1 L) and extracted with ethyl acetate, followed by washing with saturated saline solution. The organic layer was dried over magnesium sulfate. The magnesium sulfate was removed by filtration. Recrystallization was performed with ethyl acetate to obtain 4,5-bis(hydroxymethyl)-1,3-dithiol-2-thione: 1-2 in a yield o...
Embodiment 2
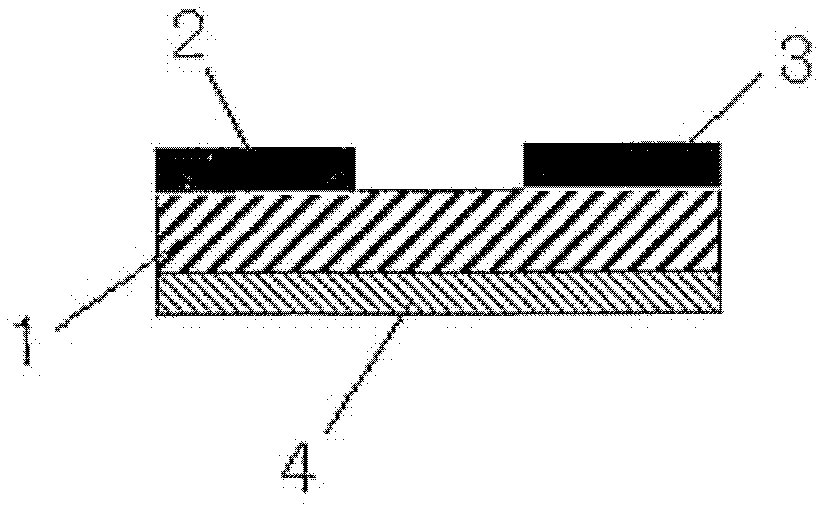
[0141] The bis(6,9-dihydro-6,9-ethylideneanthracene[2,3-d])tetrathiafulvalene synthesized in Example 1 was used by the following method: tetrathiafulvalene derivative Object (3) to produce organic membranes.
[0142] An N-type silicon substrate including a thermally oxidized film with a film thickness of 300 nm was dipped and washed in concentrated sulfuric acid for 24 hours, and then dipped in a toluene solution (1 mM) of a silane coupling agent (octyltrichlorosilane). Then, the substrate was subjected to ultrasonic treatment for 5 minutes to form a monomolecular film on the surface of the silicon oxide film.
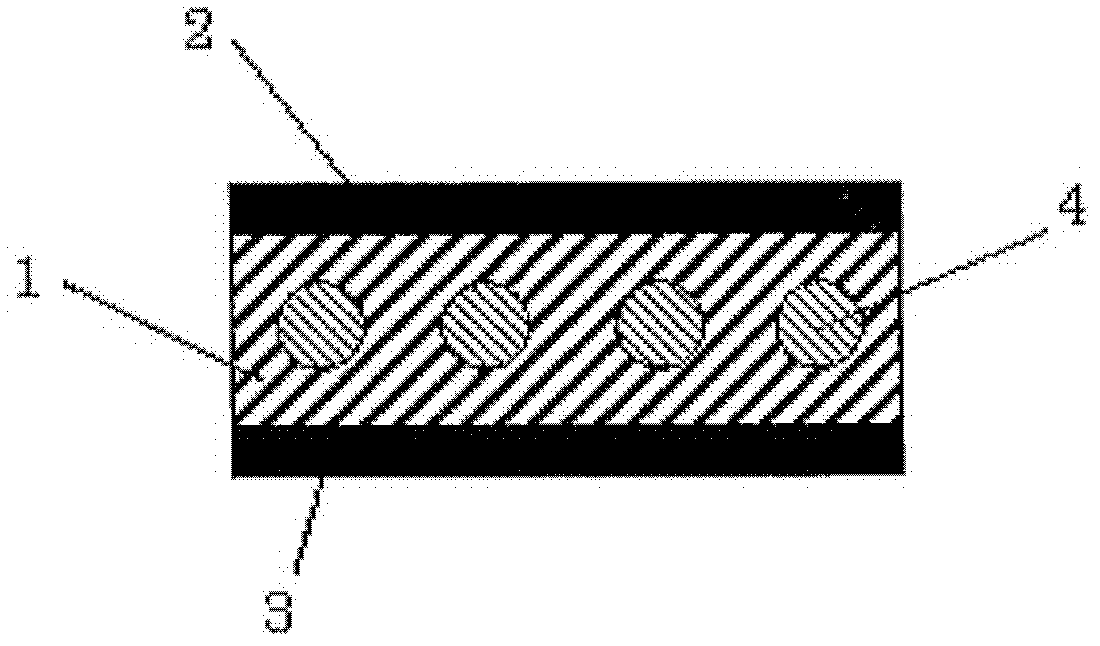
[0143] On the substrate prepared by the above method, at a back pressure of up to 10 -4 Pa, deposition rate is 0.1 And under the condition that the semiconductor film thickness is 50nm, the bis(6,9-dihydro-6,9-bridge ethylidene anthracene [2,3-d])tetrathiafulvalene obtained in Example 1:( 3) Vapor deposition is performed to obtain a smooth and uniform organic film....
Embodiment 3
[0146]
[0147] The synthetic route of compound (1) is as follows.
[0148]
[0149] Synthesis process
[0150]
[0151] The three-necked flask was purged with nitrogen, and then charged with bis(6,9-dihydro-6,9-endoethylideneanthracene [2,3-d]) tetrathiafulvalene: Tetrathiafulvalene derivation Compound (3) (0.20 g). Subsequently, the flask was placed on a hot plate set at 280°C. The yellow powder of tetrathiafulvalene derivative (3) changes into bis(anthracene[2,3-d])tetrathiafulvalene within minutes: the red powder of tetrathiafulvalene derivative (1) ( Yield: 99%).
[0152] Elemental analysis
[0153] Measured value (mass%) Calculated value (mass%)
[0154] C 71.15 71.39
[0155] H 2.77 3.20
[0156] Bis(anthracene[2,3-d])tetrathiafulvalene was analyzed by infrared spectroscopy (KBr), and the results are shown in image 3 middle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| ionization potential | aaaaa | aaaaa |
| ionization potential | aaaaa | aaaaa |
| ionization potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com