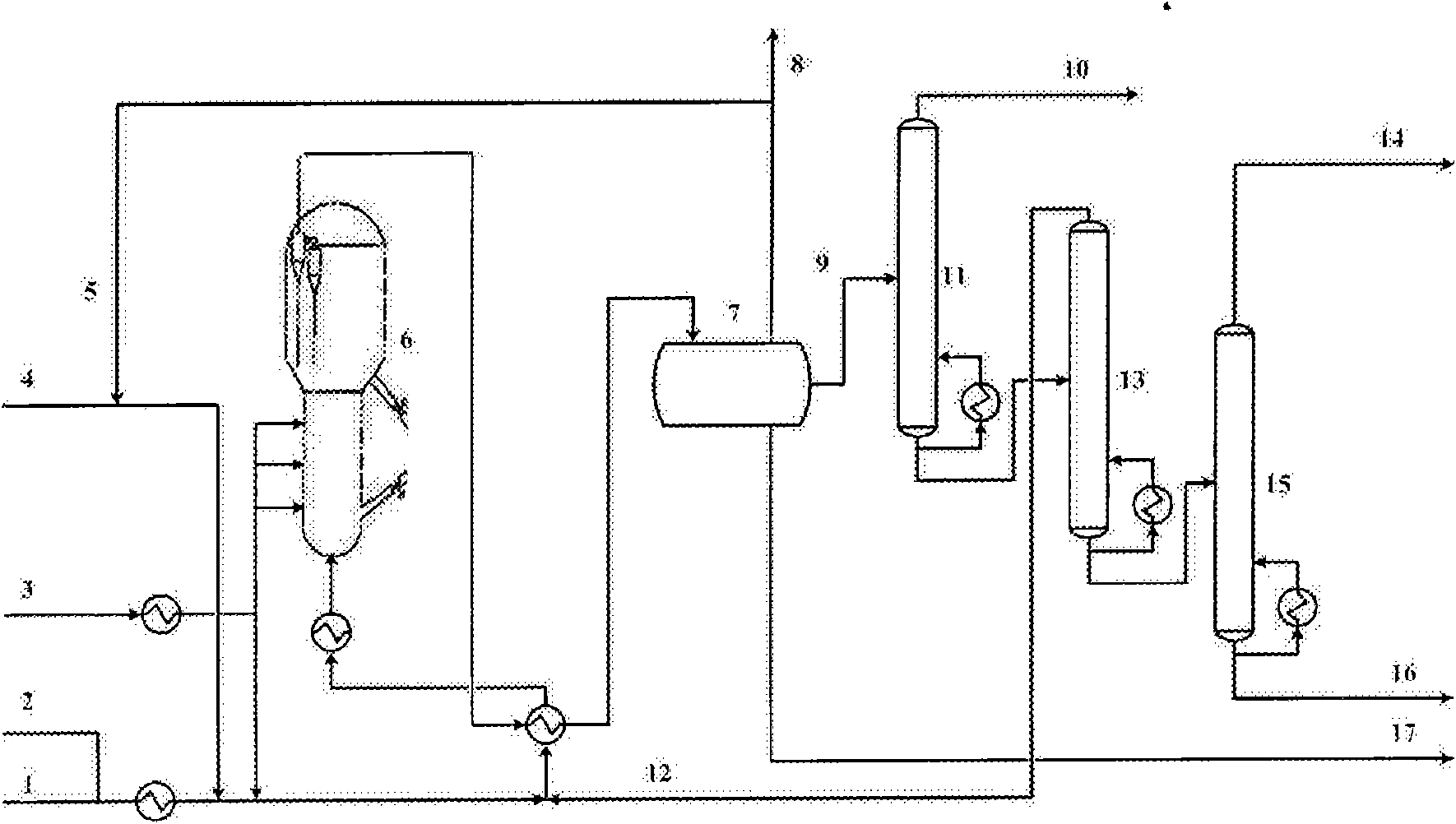
Fluidized bed method for arene methylation
A methylation and fluidized bed technology, applied in the fluidized bed field of aromatic hydrocarbon methylation, can solve the problems of large temperature rise of the reaction bed, poor catalyst stability, and many side reactions of alkylating reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
[0032] Aromatic hydrocarbon methylation was carried out according to the steps and conditions described in Comparative Example 1, wherein the alkylating agent used dimethyl ether instead of methanol, the aromatic hydrocarbon feed was a mixture of benzene and toluene, and the molar ratio of dimethyl ether to aromatic hydrocarbon was 0.25. The specific reaction conditions and evaluation results are listed in Table 1 for comparison.
[0033] Table 1
[0034]
[0035] The data in Table 1 show that, compared with methanol, the use of dimethyl ether as an alkylating agent significantly reduces the temperature rise of the catalyst bed. Using benzene and toluene as the mixed aromatics feed, although it has no effect on the control of the temperature rise of the catalyst bed, the side reaction of methanol itself and the deep alkylation reaction are still obviously suppressed, and the methyl utilization rate and xylene selectivity Significantly increased, p-xylene selectivity slight...
Embodiment 5~8
[0037] The aromatic hydrocarbon methylation reaction was carried out according to the steps and conditions described in Example 3, but the molar ratio of dimethyl ether to aromatic hydrocarbon feed was changed. The specific reaction conditions and evaluation results were listed in Table 2 for comparison.
[0038] Table 2
[0039]
[0040] The data in Table 2 shows that the amount of alkylating agent has a great influence on various performance indicators. The larger the amount is, the higher the conversion of aromatics is, but the worse the product selectivity and methyl utilization are.
Embodiment 9~14
[0042] The aromatic hydrocarbon methylation reaction was carried out according to the steps and conditions described in Example 3, and the reaction temperature, space velocity, hydrogen / aromatic hydrocarbon molar ratio and reaction pressure conditions were adjusted. The specific reaction conditions and evaluation results are listed in Table 3 for comparison.
[0043] table 3
[0044]
[0045] The data in Table 3 show that the alkylation reaction conditions have a great influence on the product distribution, and also have an influence on the temperature rise of the catalyst bed. Through the above process optimization, the selectivity of xylene can be raised up to 92.45%, the selectivity of p-xylene can be up to 89.73%, the utilization rate of methanol can be up to 82.25%, and the temperature rise can be lowered to 12.9°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com