Preparation method of antiseptic mono-benzyl-methyl fumarate
A technology of monobenzyl methyl fumarate and monomethyl fumarate, which is applied in the field of synthesis of monobenzyl methyl fumarate and can solve problems such as limited application and large irritation of DMF
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] For the esterification reaction of acid anhydride and alcohol, the productive rate is higher, without considering the use of catalysts, the test results of reaction temperature and time are as shown in table 1, and as the reaction temperature increases, the reaction speed increases gradually, reaching the point of reaction equilibrium The time is shortened, so 90°C can be selected as the suitable reaction temperature for the esterification of maleic acid monoester.
[0037] Table 1 Effect of reaction temperature and time on esterification rate
[0038]
[0039] Note: The esterification rate exceeds 100%, indicating that a diesterification reaction has occurred
Embodiment 2
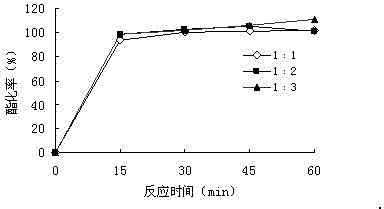
[0041] The effect of the molar ratio of maleic anhydride to benzyl alcohol on the yield of monobenzyl maleate, according to figure 1 It shows that increasing the molar ratio of reactant maleic anhydride to benzyl alcohol contributes a lot to the initial esterification rate of the reaction. The conversion rate will be close to 100%, so a 1:1 molar ratio of the reactants is selected for the reaction for 15 minutes.
Embodiment 3
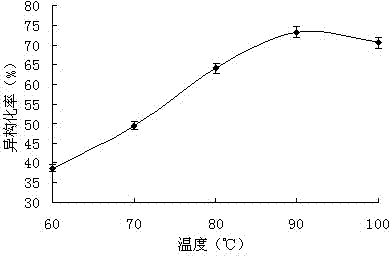
[0043] The effect of isomerization temperature on the isomerization rate Generally speaking, for the two isomers of cis and trans, the trans is more stable than the cis, because the conversion reaction of the cis isomer to the trans isomer is often Exothermic reaction. This is mainly due to the fact that the two larger groups of the cis isomer are on the same side of the double bond, which is relatively crowded, resulting in steric repulsion, so the energy is higher than that of the trans isomer, and the relative stability is poor. This can also be compared by measuring their heat of hydrogenation. The cis-isomer with a higher heat of hydrogenation has higher internal energy than the trans-isomer, so it is more active.
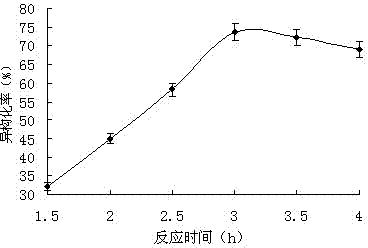
[0044] When other conditions remain unchanged, only the isomerization temperature is changed, and the yield changes as figure 2 shown. The yield increases gradually with the increase of temperature, this is because in the same reaction time, the lower react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com