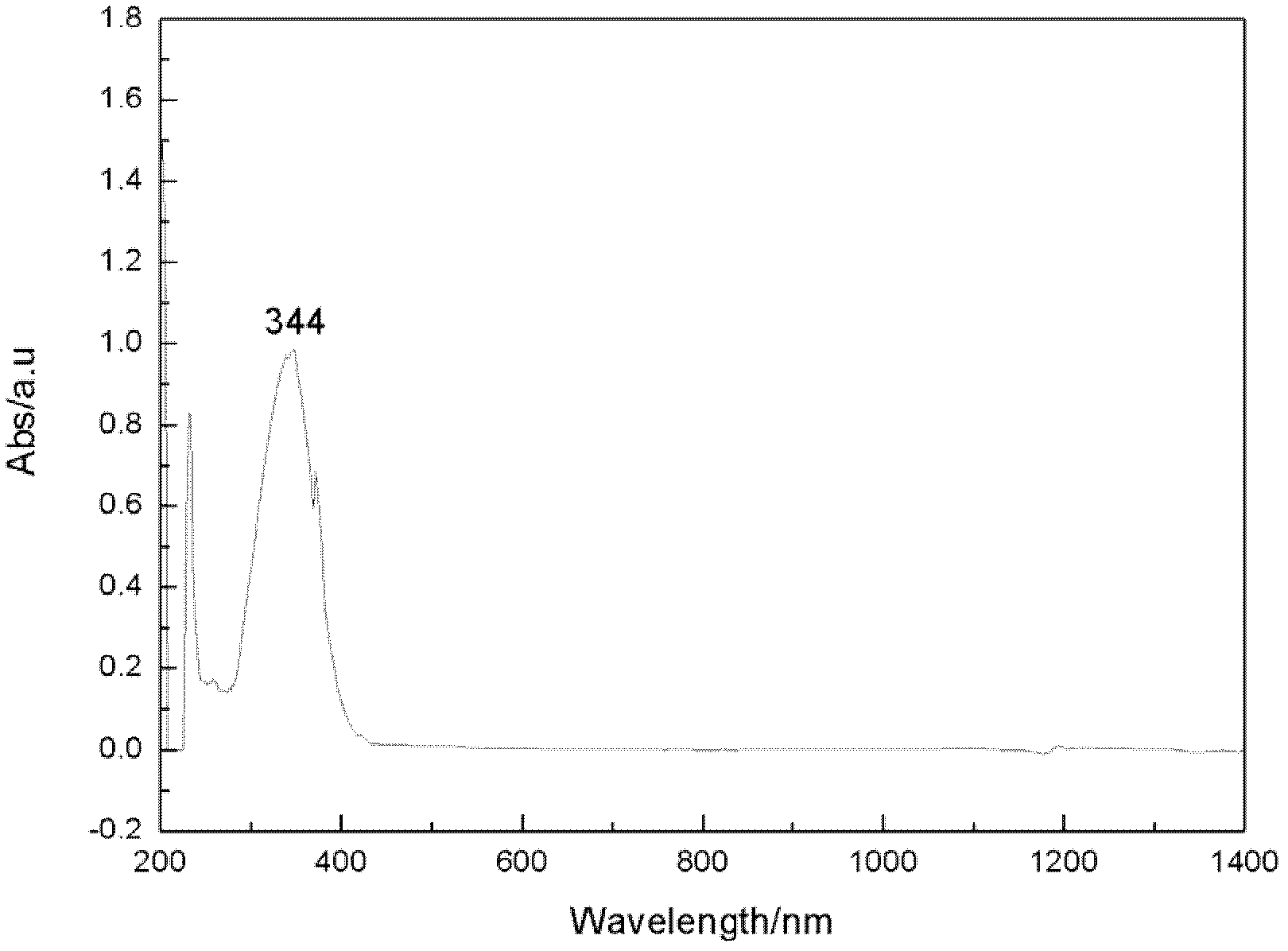
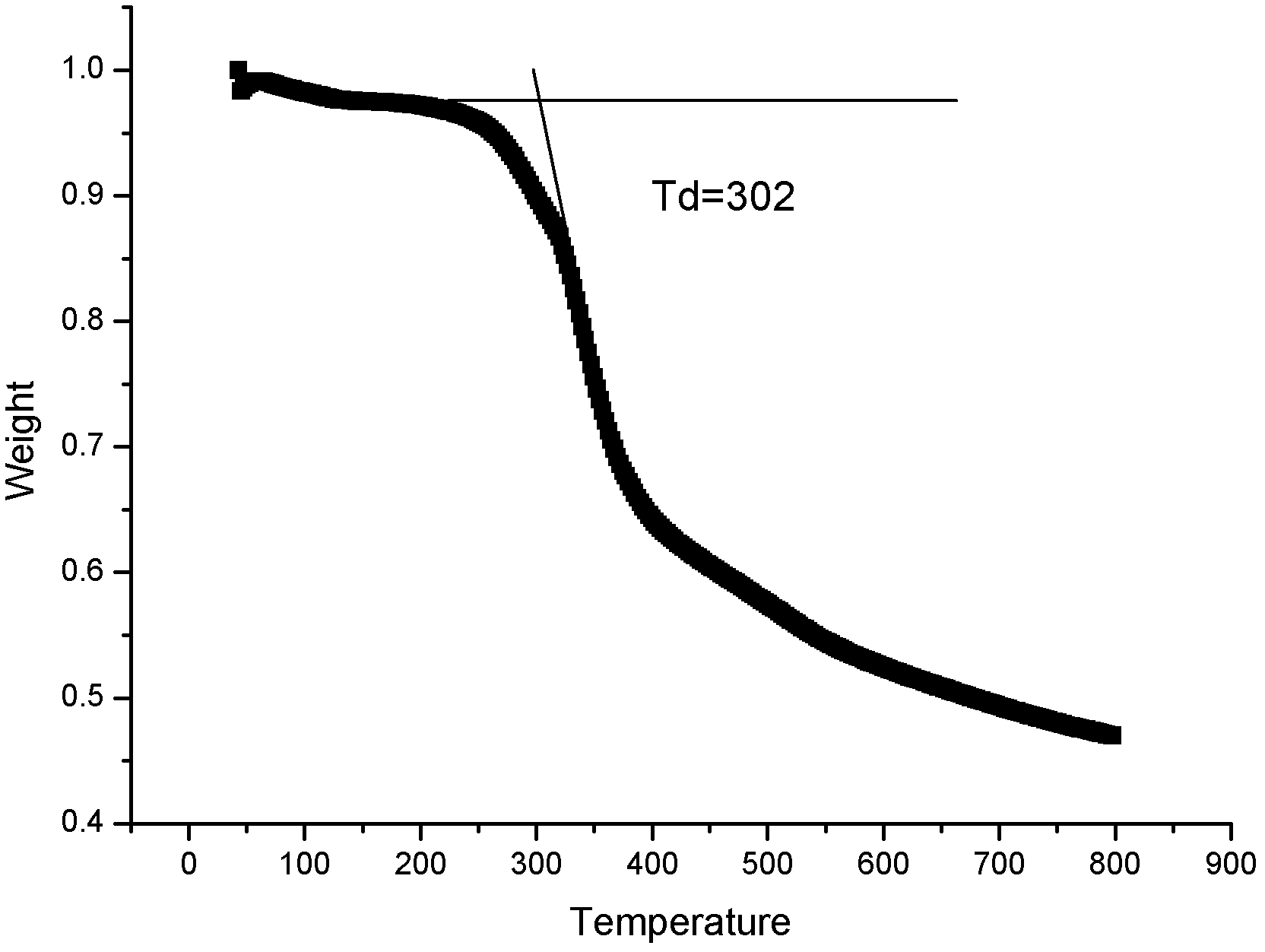
Zwitter-ion with imidazole cation and malononitrile anion structure, preparation method and application thereof
An imidazolium cation and zwitterion technology, applied in organic chemistry, nonlinear optics, optics, etc., can solve the problems of large absorption wavelength and low thermal stability, and achieve simple preparation process, high nonlinear optical coefficient, and good optical transparency. Transient effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiments 1-6 fully reflect two synthetic processes of 4-(3-methyl-1-imidazole)-2-phenylmalononitrile zwitterion; wherein, for the intermediate product 4-(3-methyl-1 The preparation of -imidazole)-2-phenylmalononitrile, two routes are given: embodiment 1,3 or embodiment 2,4.
Embodiment 7-10
[0034] Examples 7-10 fully reflect the synthesis process of 1-methyl-3-(2,6-dimethyl-4-malononitrile anionyl)phenyl-2,4,5-trimethylimidazole;
[0035] Examples 11-14 fully demonstrate the synthesis process of 1-methyl-3-(2,6-dimethyl-4-malononitrile anionyl)phenyl-2,4,5-triphenylimidazole.
[0036] Example 1: Synthesis of 1-(4-bromobenzene)-imidazole
[0037]
[0038] 8.601 g (0.050 mol) of 4-bromoaniline, 25 ml of methanol, and 7.383 g of 40% glyoxal aqueous solution (containing 0.051 mol of glyoxal) were added to a 250 ml three-necked flask, and the mixture was stirred at room temperature for 16 h. Then 5.349 g (0.100 mol) NH was added in sequence 4 Cl, 8.108 g of 37% aqueous formaldehyde solution (containing 0.100 mol of formaldehyde), 200 ml of methanol. After stirring and refluxing for 1h, 80% H was added dropwise over 10min 3 PO 4 7ml, and then continued to stir and reflux for 8h to stop the reaction.
[0039]The solvent was removed by rotary evaporation, the bla...
Embodiment 2
[0041] Example 2: Synthesis of 1-(4-iodobenzene)-imidazole
[0042]
[0043] 5.476 g (0.025 mol) of 4-iodoaniline and 12 ml of methanol were added to a 250 ml three-necked flask, and 4.2 ml of 40% glyoxal aqueous solution (containing 0.025 mol of glyoxal) was added, and the mixture was stirred at room temperature for 16 h. Then 2.675 g (0.050 mol) NH was added in sequence 4 Cl, 4 ml of 37% aqueous formaldehyde solution (containing 0.050 mol of formaldehyde), and 100 ml of methanol. After stirring and refluxing for 1h, 80% H was added dropwise in 30min 3 PO 4 3.5ml, and then continued to stir and reflux for 8h to stop the reaction.
[0044] The solvent was removed by rotary evaporation, the black residue was poured into 75 g of ice water, and the pH of the mixture was adjusted to 9 with 40% KOH solution. Extracted with dichloromethane (4×50ml), the organic phase was washed with water and saturated brine respectively, and anhydrous NaSO was added. 4 dried, filtered to r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com