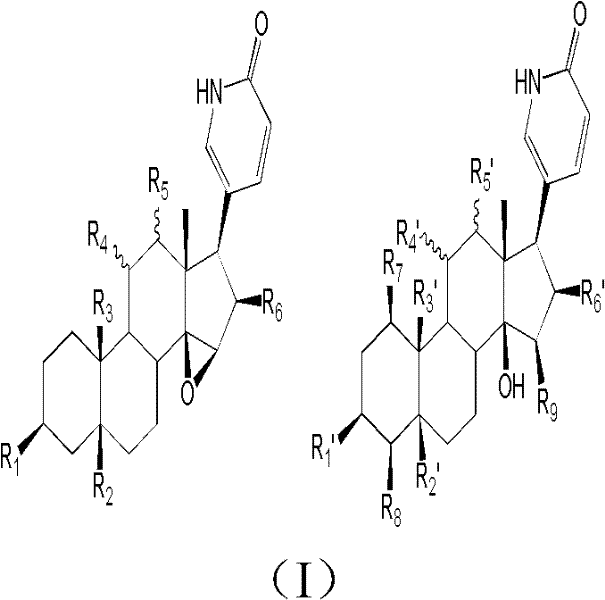
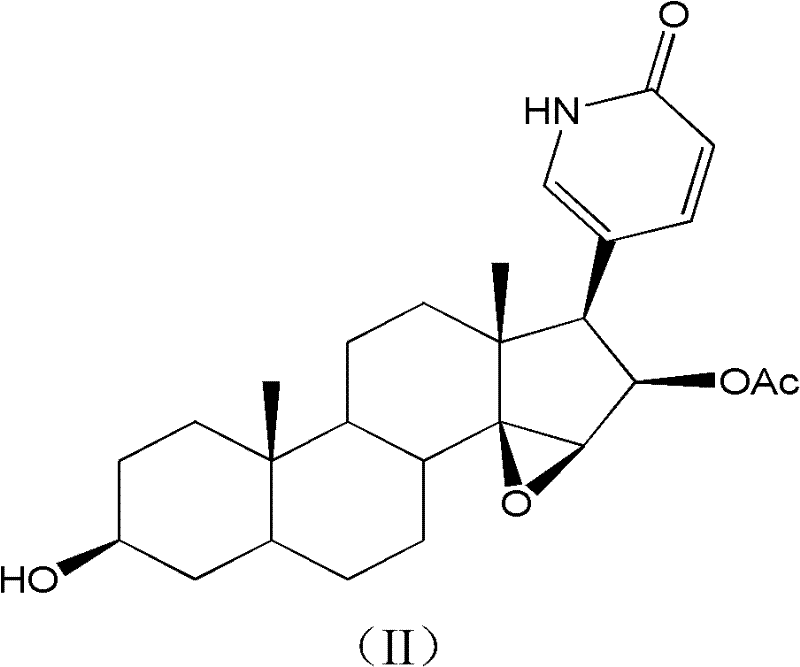
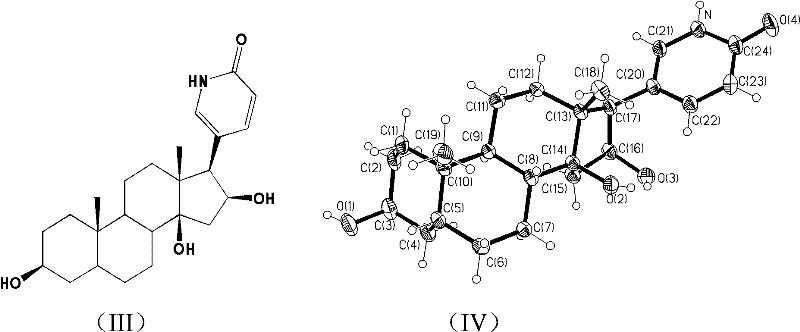
Toad lactam compound as well as preparation method and application thereof
A technology of lactam and ester compounds, which is applied in the field of bufa lactam compounds and their preparation, can solve the problems of small safety window, paralysis and death, high toxicity, etc., and achieve the advantages of convenient equipment, good inhibitory effect, and small toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of cinobufagin lactam with anti-tumor activity:
[0031] Under the protection of nitrogen, cinobufagin (separated and purified by our research group and identified its structure, please refer to Tian Haiyan for the purification steps and structural identification data, Research on the Anti-tumor Active Components of Bufo Bufa, Doctoral Dissertation of China Pharmaceutical University, 2010) 0.44g (1mmol) and ammonium acetate 0.5g (6.5mmol), dissolved in 12ml of dimethylformamide (DMF), and placed in a 100ml pressure test tube, put a stirrer, nitrogen protection seal, stirring and heating in an oil bath. React at 100°C for 3 hours, and detect the end point of the reaction with TLC; after the reaction is completed, the reaction mixture is cooled to room temperature, and the solvent DMF is removed by concentrating under reduced pressure; the sample after the reaction is dissolved in methanol, and a preparative liquid phase column (waters, C18, 5 μm, 20 × 250...
Embodiment 2
[0046] (1) Preparation of 16β-hydroxybufalin lactam with anti-tumor activity:
[0047] Under the protection of nitrogen, 16β-hydroxybufalin (separated and purified by our research group and identified its structure, please refer to Tian Haiyan for the purification steps and structural identification data, research on anti-tumor active ingredients of toadstool, doctoral dissertation of China Pharmaceutical University, 2010) 0.40g (1mmol ), ammonium acetate 0.5g (6.5mmol), DMF (12ml) are placed in a 100ml pressure-resistant test tube, put a stirrer, nitrogen protection seal, stir and heat in an oil bath, react at 140 ° C for 1.5 hours, TLC The end point of the reaction was detected; after the reaction was completed, the reaction mixture was cooled to room temperature, and the solvent DMF was removed by concentration under reduced pressure. After the reaction, the sample was dissolved in methanol, separated by a preparative liquid phase column (waters, C18, 5 μm, 20 × 250mm), the...
Embodiment 3
[0056] (1) Preparation of helleboregenin lactam with anti-tumor activity:
[0057] Under nitrogen protection, helleboregenin (separated and purified by our research group and identified its structure, see Tian Haiyan for purification steps and structural identification data, research on anti-tumor active ingredients of toadstool, doctoral dissertation of China Pharmaceutical University, 2010) 0.42g (1mmol) ), ammonium acetate 0.5g (6.5mmol), DMF (12ml) is placed in the pressure-resistant test tube of 100ml, puts into a stirring bar, nitrogen protection seals, stirs and heats in oil bath. React at 120°C for 1 hour, raise the temperature to 160°C for another 0.5 hour, and detect the end point of the reaction by TLC; after the reaction is complete, cool the reaction mixture to room temperature, and concentrate under reduced pressure to remove the solvent DMF. After the reaction, the sample was dissolved in methanol, separated by a preparative liquid phase column (waters, C18, 5 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com