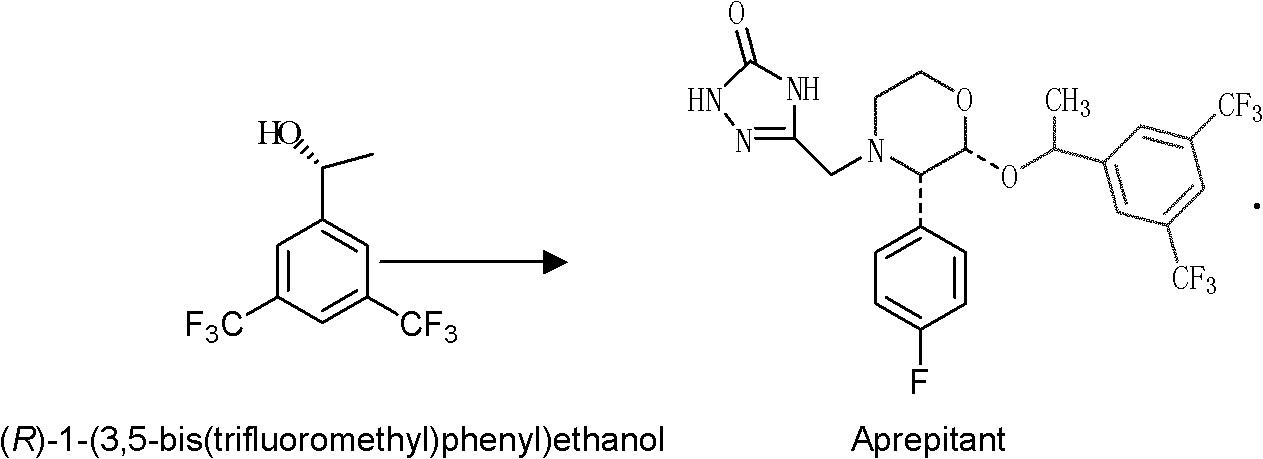
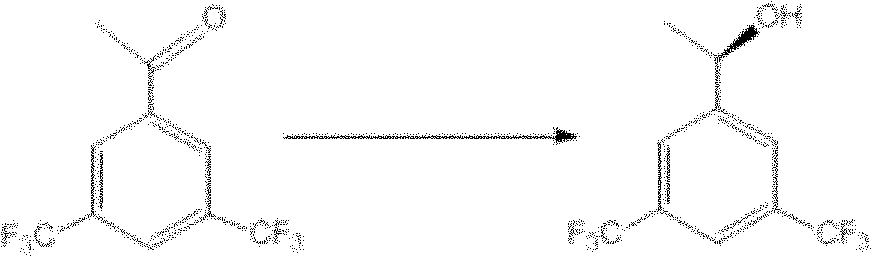Microbacterium oxydans and method for preparing chiral bis(trifluoromethyl) phenyl ethanol by using same
A technology for oxidizing microbacteria and isopropanol, applied in the direction of microorganism-based methods, biochemical equipment and methods, bacteria, etc., can solve the problems of only 15% yield, low enzyme selectivity, and low production capacity, and achieve Easy extraction and separation, high product concentration and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 strain screening
[0025] Screening of strains producing carbonyl reductase: soil samples were collected from orchards, enriched in MSM medium containing acetophenone (concentration: 1 mM), and cultured at 30° C. and 220 rpm for 7 days. 1% of the enriched solution was added to MSM medium containing acetophenone (concentration: 1 mM) for further enrichment, and culture was continued for 7 days at 30° C. and 220 rpm. The formula of MSM medium is Na2HPO42g / L, KH2PO41g / L, NH4Cl0.4g / L, MgCl20.4g / L. Dilute the second enrichment culture solution to an appropriate concentration, spread it on a screening plate (MSM+acetophenone 1mM+yeast powder 0.1g / L+agar powder 15g / L), culture at 30°C for 1-3 days, each plate Many single colonies of microorganisms can be seen on the surface, and these strains may be carbonyl reductase-producing strains. The substrate 3,5-bis-trifluoromethyl acetophenone (substrate S) was further screened for biocatalysis in order to obtain microbial ...
Embodiment 2
[0028] The identification of embodiment 2 screening bacterial strains
[0029] In order to confirm the taxonomic status of the strain and understand its performance in detail, the strain was initially identified. This strain has the following characteristics:
[0030] Colony: light yellow or yellow colony, smooth surface, neat edges, opaque.
[0031] Physiological and biochemical characteristics: Gram-positive bacteria, rod-shaped, aerobic, producing carbonyl reductase.
[0032] 16S rRNA identification: Genomic DNA was used as a template to amplify the 16S rRNA sequence with bacterial universal primers 27f and 1492r. Its sequence is as follows (1486bp):
[0033] AGAGTTTGATCCTGGCTCAGGATGAACGCTGGCGGCGTGCTTAACACATGCAAGTCGAACGGTGAACACGGAGCTTGCTCTGTGGGATCAGTGGCGAACGGGTGAGTAACACGTGAGCAACCTGCCCCTGACTCTGGGATAAGCGCTGGAAACGGCGTCTAATACTGGATATGCGACGTGATCGCATGGTCTGCGTCTGGAAAGAATTTCGGTTGGGGATGGGCTCGCGGCCTATCAGCTTGTTGGTGAGGTAATGGCTCACCAAGGCGTCGACGGGTAGCCGGCCTGAGAGGGTGACCGGCCACACTGGGACTGAG...
Embodiment 3~5
[0035] Examples 3-5 CCTCC M 2010179 growing cells transformed substrate S into product P.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com