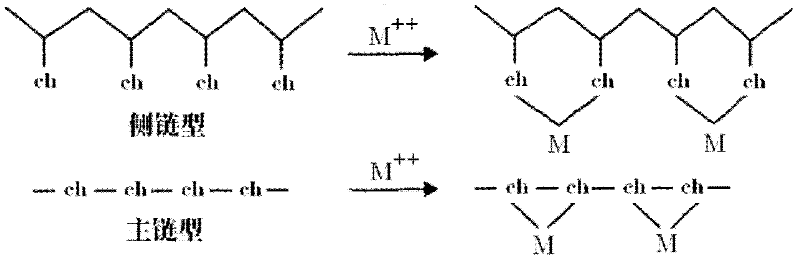
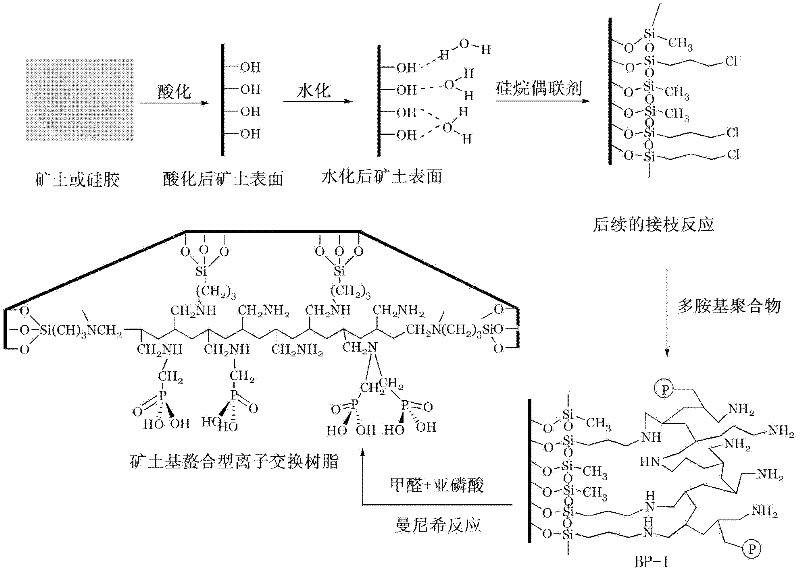
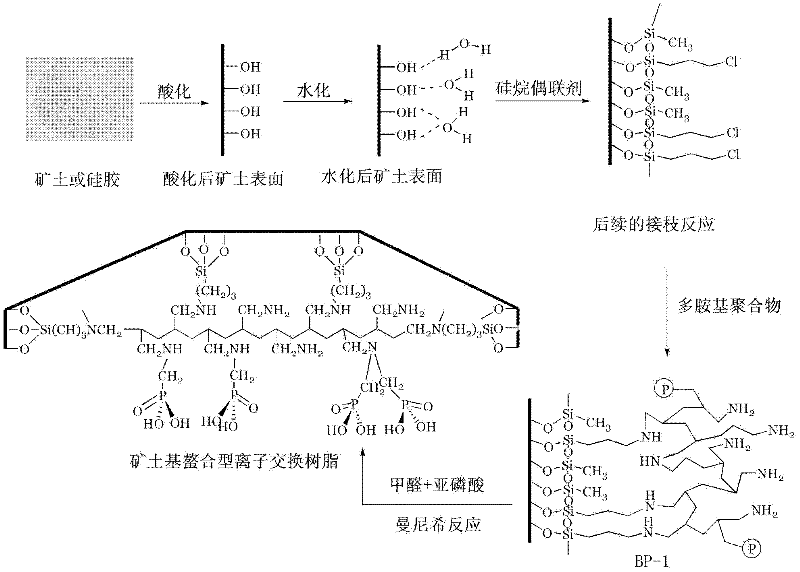
Preparation method for chelating ion exchange resin using inorganic substance as matrix
An ion exchange resin and chelating type technology, applied in the field of preparation of chelating ion exchange resin, can solve the problems of long adsorption time, high cost, low mechanical properties, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 5kg of silica gel into a corrosion-resistant reaction kettle equipped with stirring, then add 30L and 1M HCl, and after vacuuming for 15 minutes, the vacuum degree is 40mm Hg. Start heating and boiling. The stirring speed should not be too fast, and should be controlled below 70 rpm to avoid damage the silicone. Stir and pickle at a bubble point temperature of 80°C for 12 hours, then pass cooling water to cool down, and release silica gel when the temperature of the material drops to room temperature. The released silica gel was filtered to remove the filtrate, and then washed twice with deionized water and methanol successively, and the washing was stopped when the washing water showed neutrality, and the silica gel and water were separated, and then the silica gel was dried to constant weight to obtain the Silica gel (pretreated substrate) washed and dried to constant weight. Put the silica gel that has been pickled and dried to constant weight on the sand funnel...
Embodiment 2
[0038] Add 5 kg of silica gel into a corrosion-resistant reaction kettle equipped with stirring, and then add 30L, 1M HNO 3 , after evacuating for 15 minutes, the vacuum degree is 30mm Hg, start heating to 100°C and boil, the stirring speed should not be too fast, keep it below 70 rpm, so as not to damage the silica gel. Stir and pickle at a bubble point temperature of 80°C for 12 hours, then pass cooling water to cool down, and release silica gel when the temperature of the material drops to room temperature. The released silica gel was filtered to remove the filtrate, and then washed twice with deionized water and methanol in turn, and the washing was stopped when the washing water showed neutrality, and the silica gel and water were separated, and then the silica gel was dried to constant weight. Put the silica gel that has been pickled and dried to constant weight on the sand funnel, let the humid air of potassium bromide saturated solution enter the sand funnel through th...
Embodiment 3
[0043] Except that the molecular weight of polyallylamine is 50,000 instead of polyallylamine, other conditions are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com