Liquid crystal conjugated polymer of crosslinkable, fluorobenzene end cap-containing, and carbazolyl and bithienyl substitution-based difluorobenzothiadiazole, and application of liquid crystal conjugated polymer to solar cell
A technology of difluorobenzothiadiazole and conjugated polymer is applied in the field of liquid crystal conjugated polymer to achieve the effects of promoting uniform dispersion, improving stability and service life, and improving hole and electron transport rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
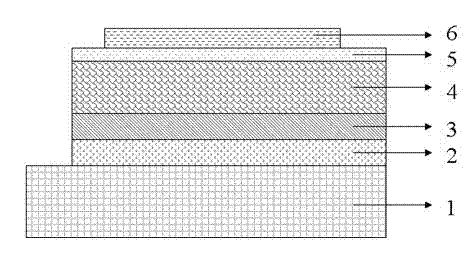
Image
Examples
Embodiment 1
[0036] Example 1: Pentafluorophenyl-terminated poly[2,7-N(heptadeca-1,16-dien-9-yl)carbazole-5,6-difluoro-4,7-bis(2- Thienyl)-2,1,3-benzothiadiazole] alternating copolymer preparation, the implementation steps are as follows.
[0037] 2,7-dibromocarbazole (7.5 g, 23.1 mmol), heptadeca-1,16-dien-9-yl-tosylate (10.3 g, 25.4 mmol), tetrabutylammonium bisulfate ( 0.15 g) was dissolved in 150 mL of acetone, and potassium hydroxide (5.1 g, 92 mmol) was added, stirred and refluxed for 2 h, cooled to room temperature, filtered, concentrated, extracted, dried, and recrystallized to obtain the product 1,[ 2,7-Dibromo-N(heptadeca-1,16-dien-9-yl)carbazole].
[0038]
[0039] 2,7-dibromo-N(heptadeca-1,16-dien-9-yl)carbazole (6.7g, 12 mmol), dipivaltanoyl diboron (7.3 g, 28.8mmol), Pd(dppf )Cl 2 (0.6 g, 1.78mmol), KOAc (8.2 g, 84mmol), a mixture of dioxane (130 mL) under argon atmosphere for 90 o C was stirred for 8 h. After cooling to room temperature, filter and concentrate, the...
Embodiment 2
[0044] Example 2: Pentafluorophenyl-terminated poly[2,7-N(trideca-1,13-dibromo-7-yl)carbazole-5,6-difluoro-4,7-bis(2- The preparation of thienyl)-2,1,3-benzothiadiazole] alternating copolymer is similar to Example 1, and the implementation steps are as follows.
[0045]
[0046] 2,7-dibromocarbazole (7.5 g, 23.1 mmol), tridecyl-1,13-dibromo-7-yl-toluenesulfonate (13.0 g, 25.4 mmol), tetrabutylammonium bisulfate ( 0.15 g) was dissolved in 150 mL of acetone, and potassium hydroxide (5.1 g, 92 mmol) was added, stirred and refluxed for 2 h, cooled to room temperature, filtered, concentrated, extracted, dried, and recrystallized to obtain the product 2,7- Dibromo-N(trideca-1,13-dibromo-7-yl)carbazole.
[0047] 2,7-dibromo-N (trideca-1,13-dibromo-7-yl) carbazole (7.9 g, 12 mmol), bis-pentanoyl diboron (7.3 g, 28.8 mmol), Pd (dppf )Cl 2 (0.6 g, 1.78mmol), KOAc (8.2 g, 84mmol), a mixture of dioxane (130 mL) under argon atmosphere for 90 o C was stirred for 8 h. After cooling ...
Embodiment 3
[0049] Example 3: Preparation of a polymer solar cell device.
[0050] 10 mg of pentafluorophenyl-terminated poly[2,7-N(heptadeca-1,16-dien-9-yl)carbazole-5,6-difluoro-4,7-bis(2-thienyl )-2,1,3-Benzothiadiazole] mixed with 30 mg PCBM, added 2 mL of chlorobenzene solution, prepared a thin film on the ITO glass modified by PEDOT:PSS by spin coating, and then vacuum evaporated Lithium fluoride and aluminum make up the cathode.
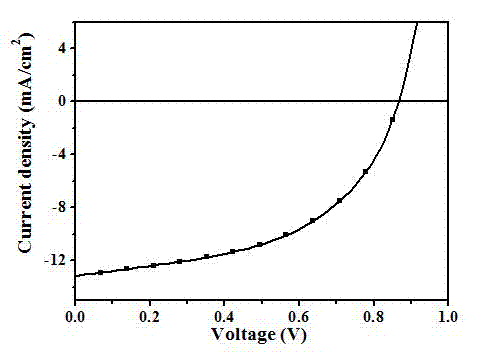
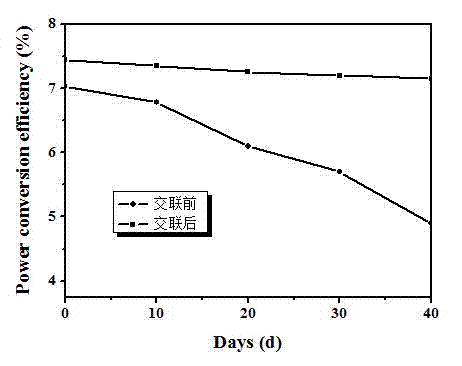
[0051] The device performance is: under the standard simulated sunlight (AM 1.5 G, 100 mW / cm2), open circuit voltage = 0.87 V; short circuit current = 13.15 mA / cm2; fill factor = 65%; energy conversion efficiency = 7.4%. Its current-voltage curve is attached as figure 2 As shown, the performance stability of the device before and after crosslinking is as follows image 3 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com