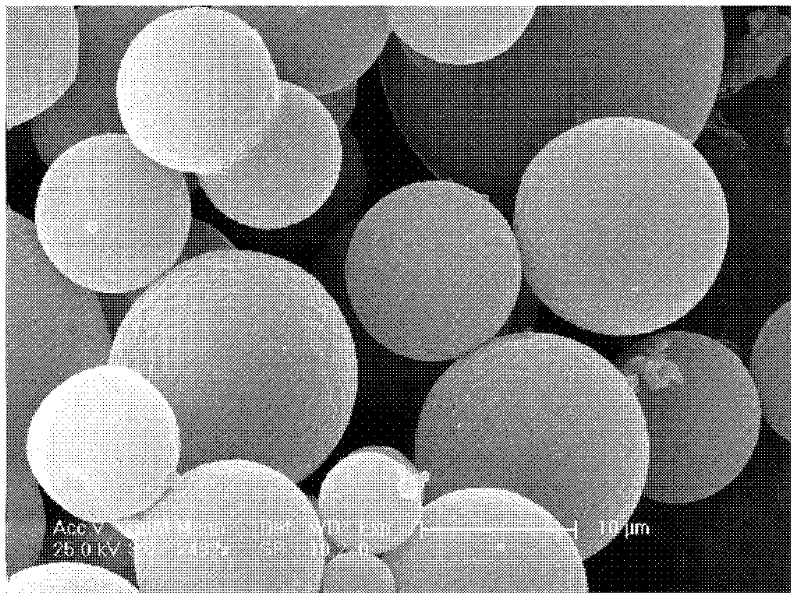
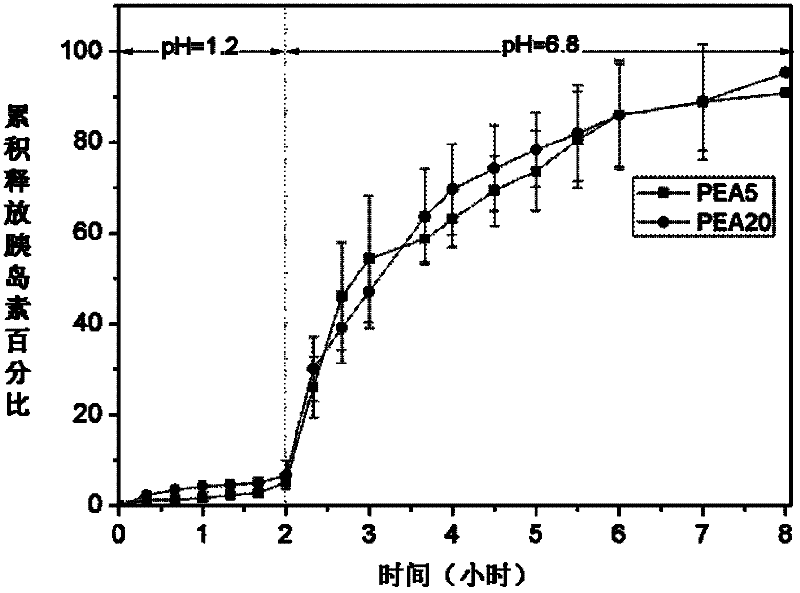
Insulin carrying microsphere and preparation method thereof
A technology of drug-loaded microspheres and insulin, which is used in pharmaceutical formulations, drug combinations, and non-active ingredients medical preparations, etc. problem, to achieve the effect of improving bioavailability, good pH sensitivity, and achieving release and absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] Correspondingly, the present invention also provides a preparation method of insulin drug-loaded microspheres, comprising the following steps:
[0042] Insulin nanoparticles are placed in the N,N-dimethylformamide (DMF) solution of the polyester amide material with the structure of formula I to form a mixed solution;
[0043] Adding the mixed solution into corn oil, forming an emulsion after shearing and stirring;
[0044] Ether is added to the emulsion, and insulin drug-loaded microspheres are obtained after extraction, and the mass percentage of the insulin nanoparticles in the insulin drug-loaded microspheres is 4-10%, preferably 5-10%, more preferably 6% ~8%,
[0045]
[0046]
[0047] Wherein, x is the molar ratio of the repeating unit of the formula II structure to the repeating unit of the formula III structure, x is 0-0.5, preferably 0.05-0.5, more preferably 0.1-0.4, more preferably 0.2-0.3, n is Polymerization.
[0048]The insulin nanoparticles used i...
Embodiment 1
[0067] After polycondensation of the monomers of the formula IV, the monomers of the formula V and the monocondensation of the formula VI, the benzyl protecting group is removed with hydrobromic acid to obtain a polyester amide polymer material with a large number of carboxyl groups in the side chain.
[0068] The specific experimental steps are:
[0069] 10 mmol, 0.5 mmol and 9.5 mmol of monomers of formula IV structure, formula V structure and formula VI structure were placed in a round bottom flask with magnetic stirring, and dry dimethyl Acetamide (DMA) was dissolved, and after the temperature was raised to 80°C, 3.2mL of dry triethylamine solution was slowly added dropwise, and after 24 hours of polycondensation, it was settled with glacial ethyl acetate and drained;
[0070] Take 1 gram of the initial product obtained by polycondensation of the three monomers and fully dissolve it in 10ml of dichloroacetic acid, add 2ml of hydrobromic acid in 33% acetic acid solution, st...
Embodiment 2
[0072] Place 10 mmol, 2 mmol and 8 mmol of monomers of formula IV structure, formula V structure and formula VI structure respectively in a round bottom flask with magnetic stirring, add dry DMA to dissolve , heated up to 80°C, slowly added dropwise 3.2ml of dry triethylamine solution, reacted for 24 hours, settled with glacial ethyl acetate, and drained;
[0073] Take 1 gram of the initial product obtained by polycondensation of the three monomers and fully dissolve it in 10ml of dichloroacetic acid, add 2ml of hydrobromic acid in 33% acetic acid solution, stir and react at room temperature for 4 hours, and settle the reactant with excess acetone out, and then repeatedly washed with ether and acetone to remove residual hydrobromic acid, and dried in a vacuum oven to obtain the polyester amide material of formula I structure, namely PEA20, and the performance parameters are shown in Table 1.
[0074] The performance parameter of the polyester amide material that table 1 embodi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com