Parathyroid hormone (PTH) derivative
A parathyroid hormone and derivative technology, applied in the fields of parathyroid hormone, hormone peptides, drug combinations, etc., can solve the problem of incomplete understanding of biological functions, improve compliance and therapeutic effect, and reduce the frequency of dosing , the effect of prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Using the pTDa expression plasmid to synthesize the sequence in SLD32 Escherichia coli strain is
[0038] Parathyroid hormone analogue of N'-SerValSerGluIleGlnLeuMetHisAsnLeuGlyArgHisLeuAsnSerMetGluArgValGluTrpLeuArgLysArgLeuGlnAspValHisAsnPhe-C'
[0039] (Arg 13,27 -PTH 1-34 )
[0040] (SEQ ID NO: 1)
[0041] 1a Construction of engineered bacteria
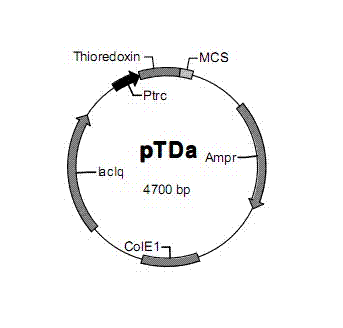
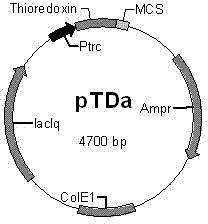
[0042] E. coli preferred codons were selected and the following cDNA sequences were designed considering the possible secondary structure to obtain high expression in the expression plasmid (pTDa):
[0043] 5’-gaattcctggttccgcgttctgtatctgaaatccaactgatgcacaacctgggtcgccacctgaactctatggaacgtgtagaatggctgcgtaaacgcctgcaggatgtacacaacttctaagtcgac-3’
[0044] (SEQ ID NO: 2)
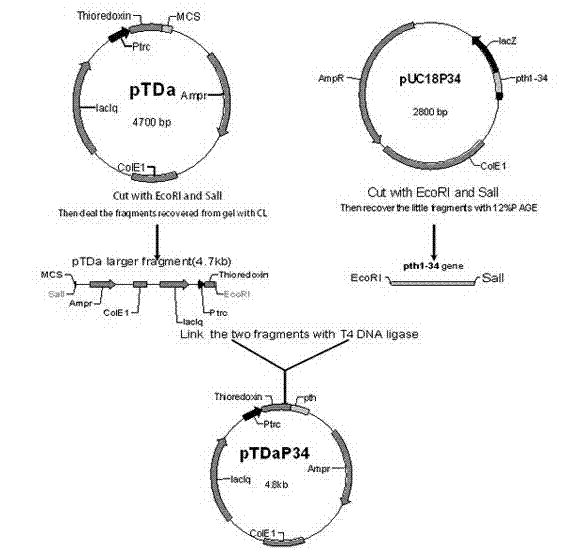
[0045] Designed Arg 13,27 -PTH 1-34 The coding gene fragment was artificially synthesized by the full sequence of Shanghai Jikang Bioengineering Co., Ltd., and loaded into the cloning vector pUC18 with EcoRI and SalI sites for preservation (pU...
Embodiment 2
[0073] Arg 13,27 Lys 26 [N-ε-(γ-Glu(N-hexadecanoyl))]-PTH 1-34 preparation of
[0074] At 0-5°C, the Arg 13,27 -PTH 1-34 4.1g (about 0.1mmol) was dissolved in 40ml of water. The pH of the solution was adjusted to 11.5 by adding 1.0M sodium hydroxide solution (3.5ml). After 5 minutes, N-methyl-2-pyrrolidone (60ml) and 1.0M acetic acid (1.5ml) were added and the temperature was maintained at 15°C . Triethylamine (0.3ml) was added followed by N-hexadecanoylglutamic acid gamma-N-hydroxysuccinimidate (146mg, 0.30mmol). After standing at 15° C. for 50 minutes, water (60 ml) was added, and 1.0 M acetic acid (2.10 ml) was added to adjust the pH of the solution to 8.5.
[0075] Yield: By analytical RP-HPLC, it was shown that the reaction mixture contained 73% (peak area) Arg 13,27 Lys 26 [N-ε-(γ-Glu(N-hexadecanoyl))]-PTH 1-34 .
[0076] The final purified product was obtained by preparative column chromatography. Dissolve the crude peptide in 90% acetonitrile aqueous soluti...
Embodiment 3
[0080] Arg 13,27 Lys 26 [N-ε-(γ-Glu-OMe(N-hexadecanoyl))]-PTH 1-34 preparation of
[0081] Under reaction conditions similar to Example 2, Arg was acylated using N-hexadecanoyl glutamate α-methyl ester γ-N-hydroxysuccinimidate 13,27 -PTH 1-34 .
[0082] Yield: By analytical RP-HPLC, it was shown that the reaction mixture contained 65% (peak area) Arg 13,27 Lys 26 [N-ε-(γ-Glu-OMe(N-hexadecanoyl))]-PTH 1-34 .
[0083] The final purified product was obtained by preparative column chromatography.
[0084] Yield: 1.4g
[0085] Molecular weight of the title compound determined by electrospray mass spectrometry: 4460 (theoretical: 4461)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com