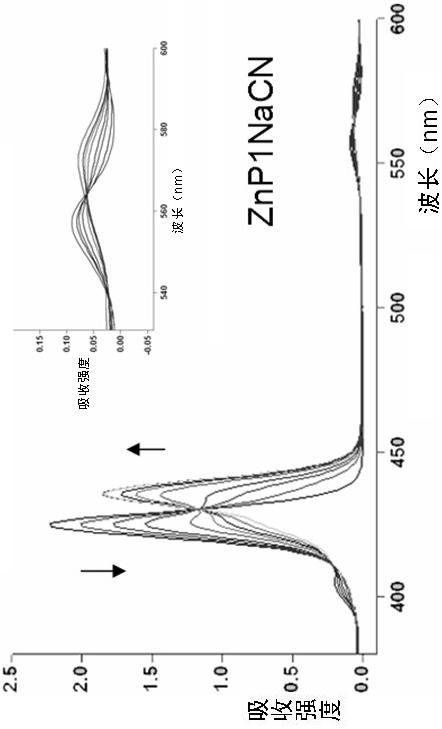
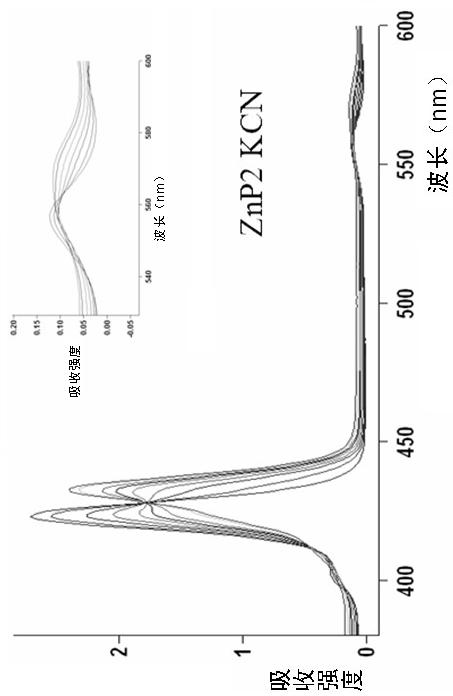
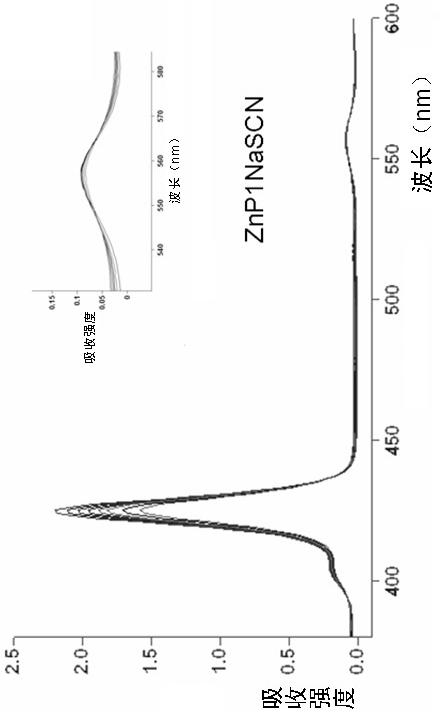
Porphyrin-crown ether compound, its synthesis and application
A technology of compound and crown ether, which is applied in the field of environmental analysis and biomedical research, can solve problems such as complex shape, large volume, and various forms, and achieve the effect of promoting solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of a zinc porphyrin-crown ether (diaza 15-crown-5) compound as an inorganic anion sensor
[0032] Step 1: Add n-octane bromide (20.7 g, 0.10 mol) to 4-carboxybenzaldehyde (15.0 g, 0.10 mol) and anhydrous K 2 CO 3 (13.8 g, 0.10 mol) in 200 mL of DMF, heated to 80°C and stirred overnight. Cool, add ethyl acetate solvent, wash with dilute KOH solution, water and saturated brine successively, anhydrous Na 2 SO 4 dry. It was filtered, concentrated, and purified by column chromatography (petroleum ether / ethyl acetate, 25:1) to obtain a white solid (23.6 g, 90%). 1 H NMR (300 M, CDCl 3 ): d 0.86 (t, J = 6.64 Hz, 3 H), 1.28-1.46 (m, 10 H), 1.73-1.84 (m, 2 H), 4.35 (t, J = 6.68 Hz, 2 H), 7.95 (d, J = 8.10 Hz, 2 H), 8.19 (d, J = 8.10 Hz, 2 H), 10.10 (s, 1 H).
[0033] The second step: the product of the first step (8.70 g, 33.2 mmol) and pyrrole (23 ml, 10 equiv.) in 250 mL toluene solution was protected with nitrogen for 30 min, and then 1 mL of p-tol...
Embodiment 2
[0036] Example 2: Synthesis of a zinc porphyrin-crown ether (diaza 18-crown-6) compound as an inorganic anion sensor
[0037] Step 1: Add n-heptane bromide (19.3 g, 0.10 mol) to 4-carboxybenzaldehyde (15.0 g, 0.10 mol) and anhydrous K 2 CO 3 (13.8 g, 0.10 mol) in 200 mL of DMF, heated to 80°C and stirred overnight. Cool, add ethyl acetate solvent, wash with dilute KOH solution, water and saturated brine successively, anhydrous Na 2 SO 4 dry. It was filtered, concentrated, and purified by column chromatography (petroleum ether / ethyl acetate, 25:1) to obtain a white solid (22.1 g, 90%). 1 H NMR (300 M, CDCl 3 ): d 0.86 (t, J = 6.64 Hz, 3 H), 1.28-1.46 (m, 10 H), 1.73-1.84 (m, 2 H), 4.35 (t, J = 6.68 Hz, 2 H), 7.95 (d, J = 8.10 Hz, 2 H), 8.19 (d, J = 8.10 Hz, 2 H), 10.10 (s, 1 H).
[0038] The second step: the product of the first step (7.58 g, 33.2 mmol) and pyrrole (23 ml, 10 equiv.) in 250 mL of toluene solution was protected by nitrogen for 30 min, and then 1 mL of ...
Embodiment 3
[0041] Example 3: Referring to Example 1, the corresponding zinc porphyrin-crown ether compound can be obtained only by replacing brominated n-octane with C1-C7, C9 or C10 brominated n-alkanes or brominated benzyl. Likewise, magnesium, nickel or manganese porphyrin-crown ethers can be obtained by substituting magnesium acetate, nickel acetate or manganese acetate for zinc acetate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com