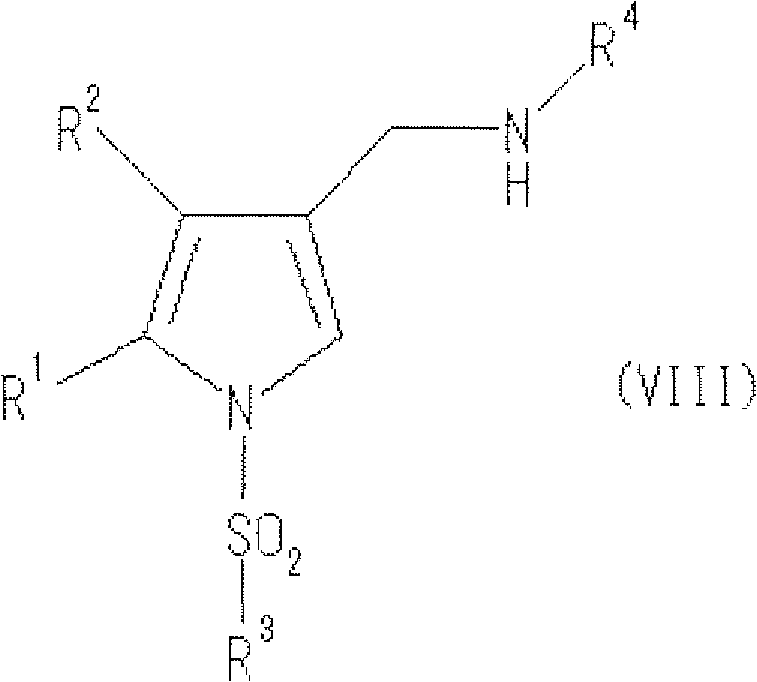
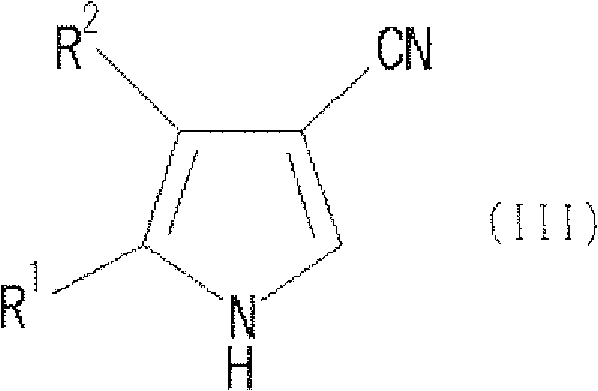
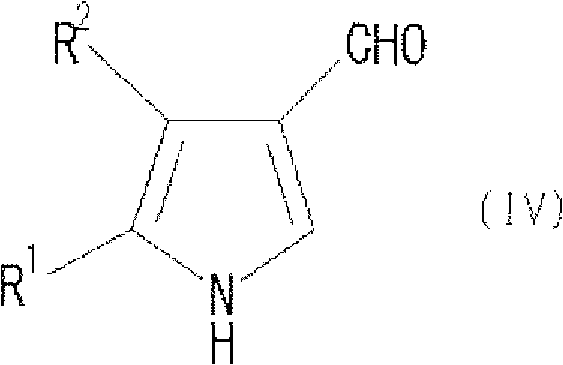
Process for producing pyrrole compound
A compound and product technology, applied in the field of preparing pyrrole compounds, can solve problems such as desulfurization reaction of 2-mercaptopyrrole derivatives that have not been described
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0294] The preparation method of the present invention is described in detail below.
[0295] As the salts of compounds (I)-(XVII) in the reaction, there may be mentioned metal salts, ammonium salts, salts with organic bases, salts with inorganic bases, salts with organic acids, and alkali or Salts of acidic amino acids, etc. Examples of preferable metal salts include alkali metal salts such as sodium salts, potassium salts, etc., alkaline earth metal salts such as calcium salts, magnesium salts, barium salts, etc., aluminum salts, and the like. Examples of preferred salts with organic bases include trimethylamine, triethylamine, pyridine, picoline, 2,6-lutidine, ethanolamine, diethanolamine, triethanolamine, cyclohexylamine, dicyclohexylamine , N, N'-dibenzylethylenediamine and the like. Examples of preferable salts with inorganic acids include salts with hydrochloric acid, hydrobromic acid, nitric acid, sulfuric acid, phosphoric acid and the like. Examples of preferred sa...
Embodiment 1
[0469] 5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile
[0470] 2-Chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile (5.0 g, 22.7 mmol), methanol (150 ml) and diisopropylethylamine (3.8 g, 29.5 mmol) were added to high pressure autoclave and purged the autoclave with nitrogen. 5% palladium on carbon (N.E. CHEMCAT, Standard, 0.5 g) was added. Then, the mixture was vigorously stirred at an internal temperature of 15-25° C. for about 4 hours under a hydrogen atmosphere (0.1 MPa). After purging with nitrogen, the catalyst was filtered off and washed with methanol (15ml). The organic layer was concentrated to about 13 g under reduced pressure. The amount of the contents was adjusted to about 28 g with ethanol. Water (40 ml) was added dropwise at an internal temperature of 15-25°C, and the mixture was stirred at the same temperature for 1 hr. The mixture was cooled to an internal temperature of 0-10°C and stirred for 1 hour. The precipitated crystals were collected by filtration...
Embodiment 2
[0477] 5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile
[0478] Add 2-chloro-5-(2-fluorophenyl)-1H-pyrrole-3-carbonitrile (25.0g, 113mmol), ethanol (350ml) and diisopropylethylamine (19.0g, 147mmol) into the autoclave , the autoclave was purged with nitrogen. A suspension of 5% palladium on carbon (N.E. CHEMCAT, Standard, 2.5 g) in ethanol (25 ml) was added. Under a hydrogen atmosphere, the mixture was stirred vigorously at an internal temperature of 15-25°C for about 7 hours. After purging with nitrogen, the catalyst was filtered off and washed with ethanol (75ml). The filtrates were combined and concentrated under reduced pressure to approximately 140 g. Water (200 ml) was added dropwise at an internal temperature of 20-30°C, and the mixture was stirred at the same temperature for 0.5 hr. The mixture was cooled to an internal temperature of 0-10°C and stirred for 1 hour. The precipitated crystals were collected by filtration, washed with a cold mixed solution of ethanol an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com