Fluorescence-labeled O6-benzyl guanine and preparation and application thereof
A technology of benzylguanine and fluorescent labeling, applied in fluorescence/phosphorescence, biochemical equipment and methods, luminescent materials, etc., can solve the complex preparation process of labeled DNA and labeled oligonucleotides, difficult long-term storage, and membrane permeability gender issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
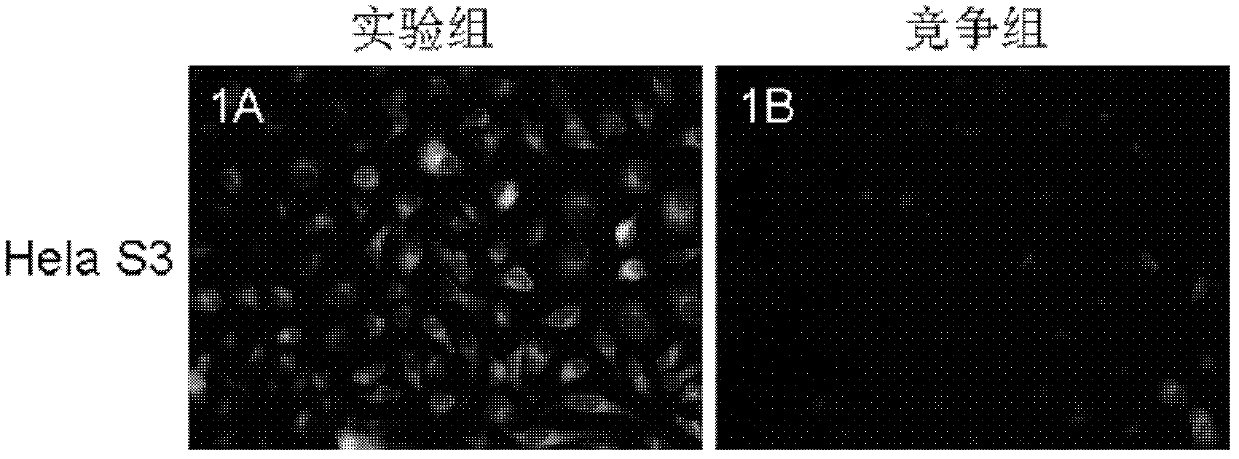
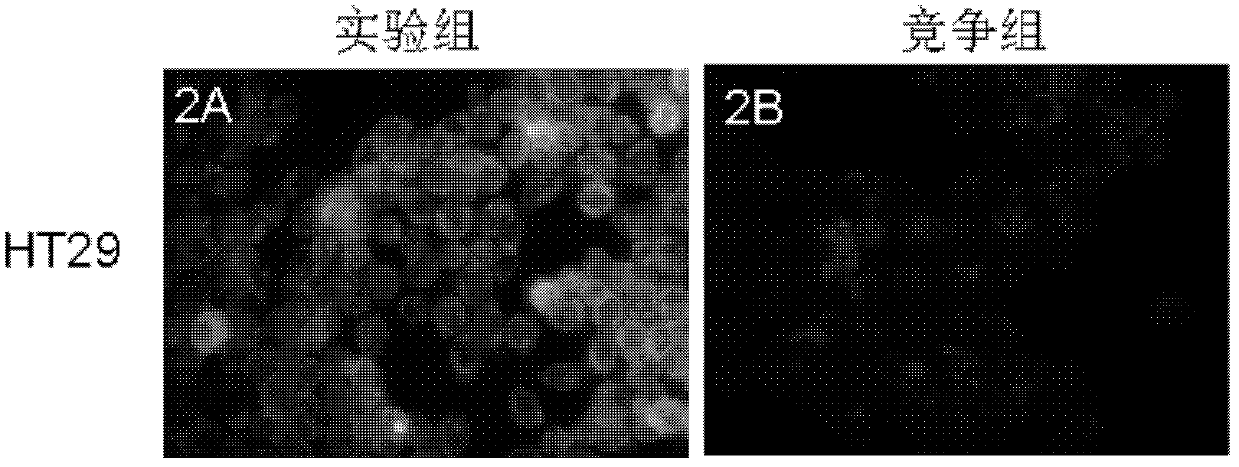
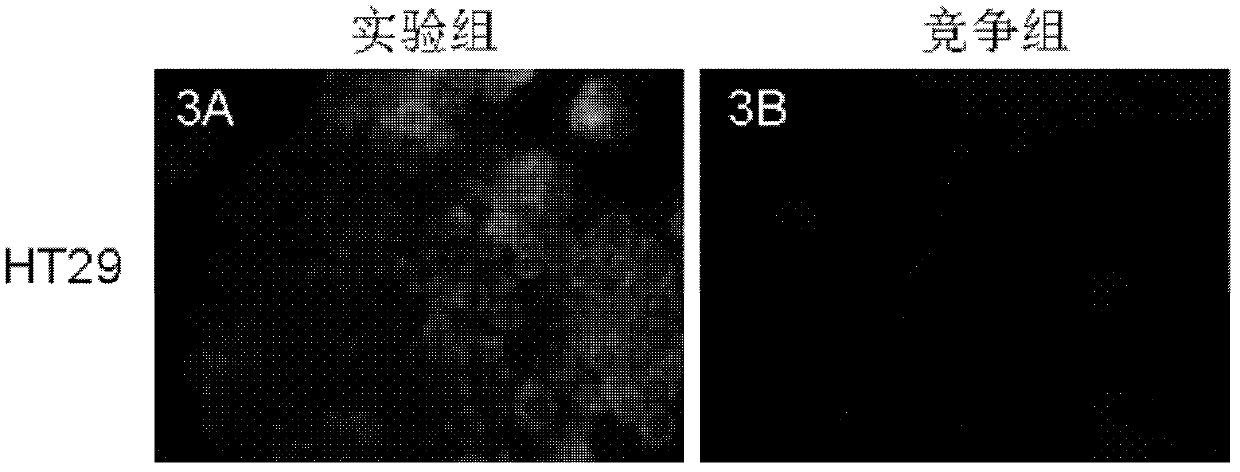
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of ethyl 4-((2-(2-(2-azidoethoxy) ethoxy) ethoxy) methyl) benzoate (IIc)
[0041] Weigh 2-(2-(2-azidoethoxy)ethoxy)ethanol (1.060 g, 6 mmol), dissolve in dry tetrahydrofuran (25 mL), add 1 equivalent of NaH, stir at room temperature for 5 minutes, add 1 Equivalent of ethyl p-chloromethylbenzoate (1.200 g, 6 mmol), continued to react at room temperature for 3 hours, quenched with water (10 mL), the crude product was extracted with ethyl acetate (50 mL), and the organic phase was Dry over anhydrous sodium sulfate for 10 minutes, concentrate, and purify by silica gel column chromatography, eluting with petroleum ether / ethyl acetate (3:1), to obtain a colorless oil (0.950 g, 2.8 mmol), with a yield of 47% .
[0042] δ H (500 MHz, CDCl 3 ) 8.02 (2 H, d, J =8.0), 7.41 (2 H, d, J =8.0), 4.63 (2 H, s), 4.37 (2 H, q, J =7.1), 3.73 – 3.64 (10 H, m), 3.38 (2 H, t, J =5.0), 1.39 (3 H, t, J =7.1).
Embodiment 2
[0043] Example 2: Preparation of ethyl 3-((2-(2-(2-azidoethoxy)ethoxy)ethoxy)methyl)benzoate (IIh)
[0044] Weigh 2-(2-(2-azidoethoxy)ethoxy)ethanol (1.260 g, 7.2 mmol), dissolve in dry tetrahydrofuran (35 mL), add 1 equivalent of NaH, stir at room temperature for 5 minutes, add 1 equivalent of ethyl m-bromomethylbenzoate (1.750 g, 7.2 mmol), continued to react at room temperature for 3 hours, quenched with water (10 mL), the crude product was extracted with ethyl acetate (50 mL), and the organic phase After drying over anhydrous sodium sulfate for 10 minutes, it was concentrated and purified by silica gel column chromatography, eluting with petroleum ether / ethyl acetate (3:1), to obtain a colorless oil (1.400 g, 4.1 mmol), with a yield of 58 %.
[0045] δ H (500 MHz, CDCl 3 ) 8.01 (1 H, s), 7.96 (1 H, d, J =7.7), 7.56 (1 H, d, J =7.7), 7.42 (1 H, t, J =7.7), 4.61 (2 H, s), 4.38 (2 H, q, J= 7.1), 3.72 – 3.65 (10 H, m), 3.38 (2 H, t, J =5.1), 1.40 (3 H, t, J =...
Embodiment 3
[0046] Example 3: Preparation of 4-(2-(2-(2-(trifluoroacetamido)ethoxy)ethoxy)ethoxy)methyl-benzyl alcohol (IIIc)
[0047] Weigh 4-((2-(2-(2-azidoethoxy)ethoxy)ethoxy)methyl)ethyl benzoate (0.950 g, 2.8 mmol), dissolve in dry tetrahydrofuran (40 ml ), add 1.5 equivalents of LiAlH 4 (0.160 g, 4.2 mmol), the system was stirred and reacted under reflux for 3 hours, then the heating was stopped, and after it was naturally cooled to room temperature, it was quenched by adding water (5 mL), and the product was extracted with ethyl acetate (50 mL), acetic acid The ethyl ester phase was washed once with saturated brine (10 mL), dried by adding anhydrous sodium sulfate for 10 minutes, filtered, and the filtrate was concentrated under reduced pressure to obtain a light yellow oil. The oil was then dissolved in absolute ethanol (20 mL), and 1 equivalent of triethylamine (0.280 g, 2.8 mmol) and 1 equivalent of ethyl trifluoroacetate (0.400 g, 2.8 mmol) were added successively, and the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com