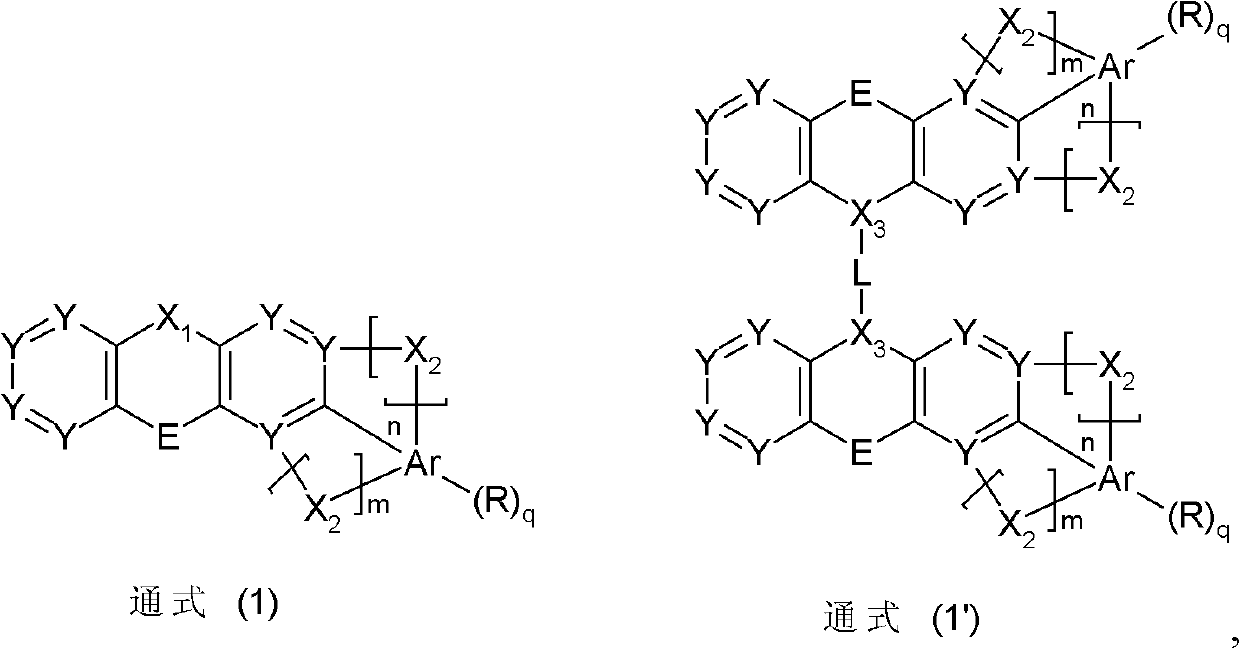
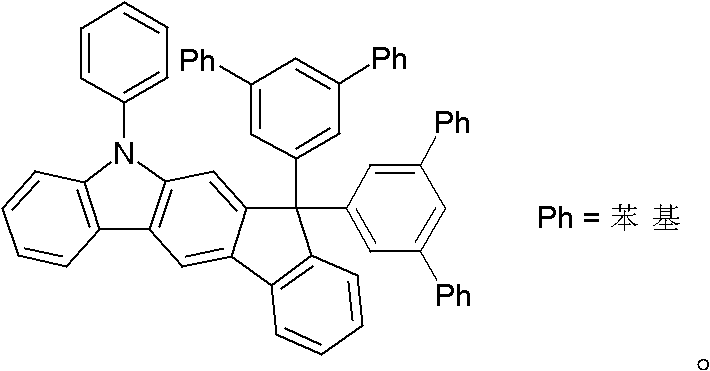
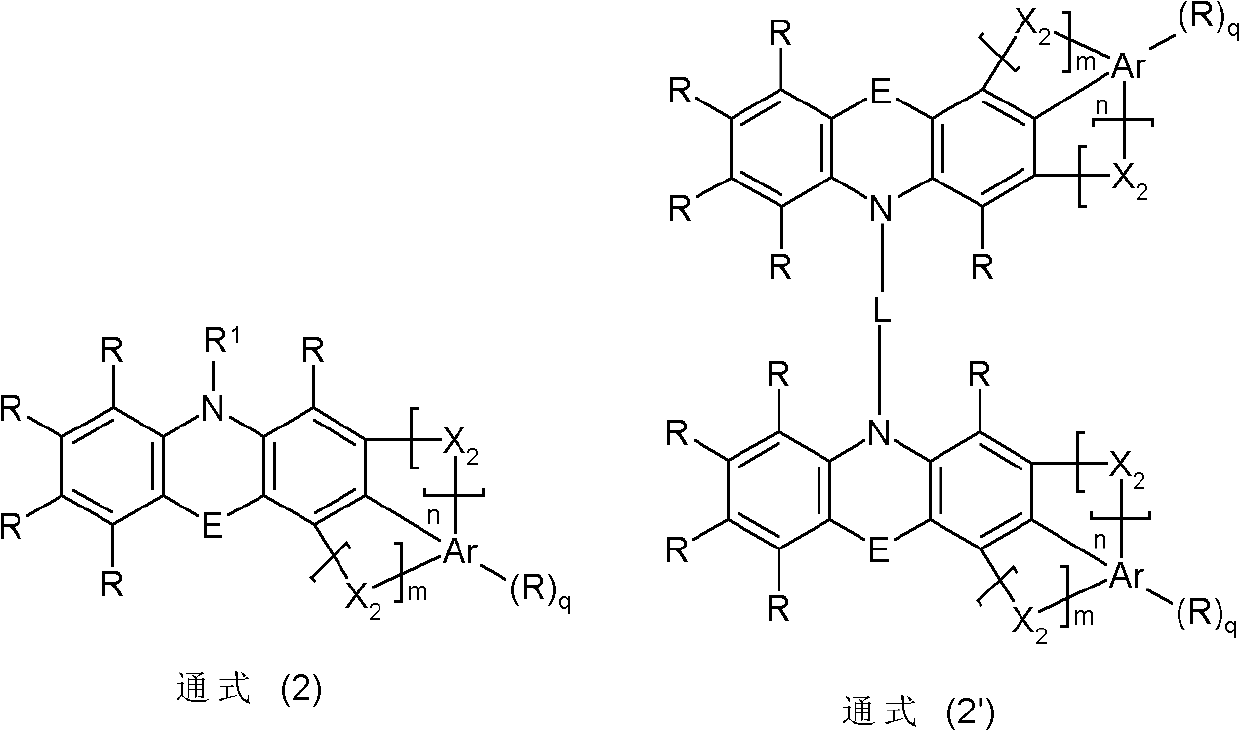
Materials for organic electroluminescent devices
A technology of general formula and compound, applied in the field of preparing the compound of the present invention, can solve the problems of short working life, vapor deposition, low electron mobility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] Example 1: Synthesis of 2,5-bis(9-phenyl-9H-carbazol-3-yl) diethyl terephthalate:
[0230]
[0231] 913mg (3mmol) of tris(o-tolyl)phosphine, and then 112mg (0.5mmol) of palladium(II) acetate were added to 18.6g (49.1mmol) of 2,5-dibromoterephthalate diethyl and 38g which were well stirred (102mmol) (9-phenyl-9H-carbazol-3-yl) boric acid and 51g (221mmol) tripotassium phosphate in 380ml toluene, 190ml two In a suspension of a mixture of alkane and 480 ml of water, the mixture was heated to reflux for 16 h. After cooling to room temperature (RT), the precipitated solid was filtered off with suction, washed three times with 50 ml of toluene, three times with 50 ml of ethanol: water (1:1, V: V), and three times with 100 ml of ethanol, and removed from DMF (About 10ml / g) Recrystallize three times.
[0232] Yield: 22.6 g (32 mmol), 63.0%.
Embodiment 2
[0233] Example 2: Synthesis of 2-[4-(1-hydroxy-1-methylethyl)-2,5-bis(9-phenyl-9H-carbazol-3-yl)phenyl]propan-2- alcohol:
[0234]
[0235] Dissolve 6.8g (9.67mmol) 2,5-bis(9-phenyl-9H-carbazol-3-yl)diethyl terephthalate in 40ml THF, add 40ml (42mmol) at -75℃ A 2M methyl lithium solution in diethyl ether, the mixture was stirred at -75°C for 3 h. After warming to room temperature, use NH 4 The mixture was hydrolyzed with Cl solution, extracted with ethyl acetate, dried, and the solvent was removed under reduced pressure. The remaining colorless solid was recrystallized twice from toluene / EtOH, leaving 4.2 g (6.2 mmol) of 62% diol in the form of colorless crystals.
Embodiment 3
[0237]
[0238] 3.38g (5mmol) 2-[4-(1-hydroxy-1-methylethyl)-2,5-bis(9-phenyl-9H-carbazol-3-yl)phenyl]propan-2 -The alcohol is dissolved in 30 ml of dichloromethane and cooled to 5°C, and a mixture of 4 g of polyphosphoric acid in 3 ml of methanesulfonic acid is added. After keeping at 5°C for 30 minutes, 50 ml of EtOH was added and the mixture was heated at boiling for 1 h. The colorless precipitate was filtered off, washed twice with EtOH and heptane, and recrystallized from chlorobenzene once to obtain a colorless solid product (2 g, 4 mmol) with a yield of 75% and a purity of >99.9% according to RP-HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com