Method of expanding human hepatocytes in vivo
A technology of hepatocytes and liver, applied in the field of hepatocyte expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
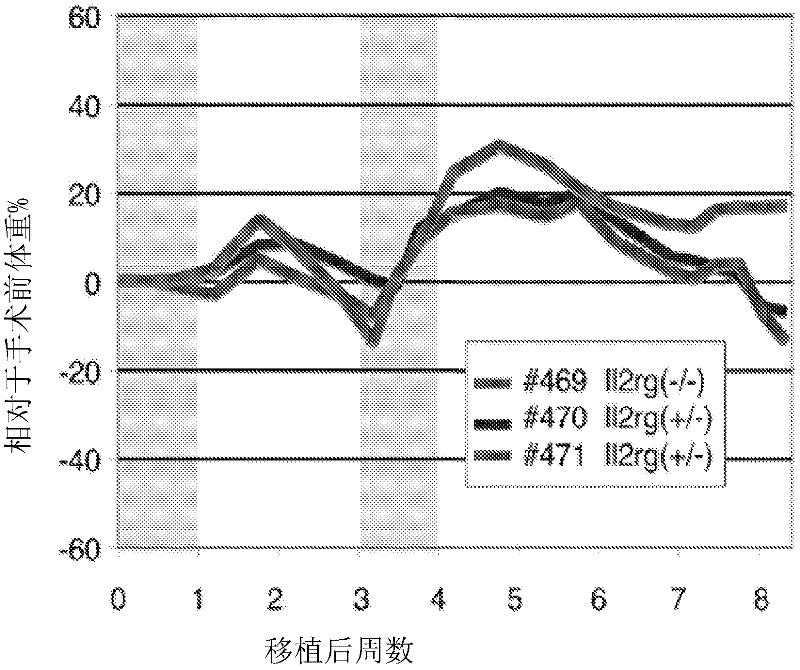
[0284] Example 1: Fah - / - / Rag2 - / - / Il2rg - / - Generation of (FRG) mice
[0285] A number of different strategies can be applied to generate immunodeficient mice, including, for example, by administering immunosuppressive drugs or by introducing one or more genetic alterations. This example describes the generation of genetically altered immunodeficient mice.
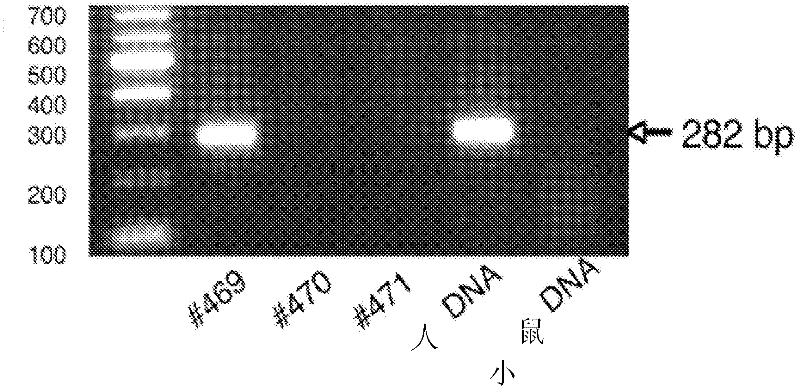
[0286] To generate immunodeficient Fah that completely lacks T cells, B cells, and NK cells, but does not have DNA repair defects - / - mouse strain that produced the Fah - / - / Rag2 - / - / Il2rg - / - (FRG) mice. Will the male Fah - / - 129S4 mice (Grompe et al., Genes Dev. 7:2298-2307, 1993) with female Rag2 - / - / Il2rg - / - Mice (Taconic) were crossed. All animals were maintained with drinking water containing 2-(2-nitro-4-trifluoromethyl-benzoyl)-1,3-cyclohexanedione (NTBC) at a concentration of 1.6 mg / L (Grompe et al ., Nat. Genet. 70:453-460, 1995). To confirm the genotype of each animal, PCR-based genotyping was...
Embodiment 2
[0288] Example 2: Histology and Engraftment Detection Analysis
[0289] Histology and Immunocytochemistry
[0290] FAH immunohistochemistry was performed as previously described (Wang et al., Am. J. Pathol. 161:565-574, 2002). Briefly, liver and kidney tissues fixed in 10% phosphate-buffered formalin at pH 7.4, dehydrated in 100% ethanol, and embedded in paraffin at 58 °C. Deparaffinized 4 μm sections were stained with hematoxylin and eosin. For immunohistochemistry, wash sections with 3% H 2 o 2 treated with methanol solution to block endogenous peroxidase. Prior to incubation with the primary antibody, avidin and biotin blocking was also performed. Sections were incubated with anti-FAH rabbit antibody or HepPar antibody (DAKO) for 2 hours at room temperature. Then incubated with HRP-conjugated secondary antibody. Signals were detected by diaminobenzidine (DAB).
[0292] Fumaryl acetoacetate was incubated with the cytoplasmic liver fraction ...
Embodiment 3
[0308] Example 3: Isolation and Cryopreservation of Human Hepatocytes
[0309] Human hepatocytes were isolated from donor livers not used for liver transplantation following a previously described procedure (Strom et al., Cell Transplant. 15:S 105-110, 2006). Briefly, liver tissue was perfused with Hanks' balanced salt solution (Cambrex) without calcium and magnesium supplemented with 0.5 mM EGTA (Sigma) and HEPES (Cellgro), and then treated with Eagle's minimal essential medium (Cambrex). 100mg / L collagenase XI (Sigma) and 50mg / L deoxyribonuclease I (Sigma) were digested through the existing vasculature. The cells were washed 3 times with Eagle's minimal essential medium supplemented with 7% bovine serum (Hyclone) at 50 x g for 2 minutes each. Transfer pelleted hepatocytes to cold VIASPAN TM Medium (universal active pulse wash and cold storage solution for preservation of intra-abdominal organs; also known as University of Wisconsin solution or UW).
[0310] Transport the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com