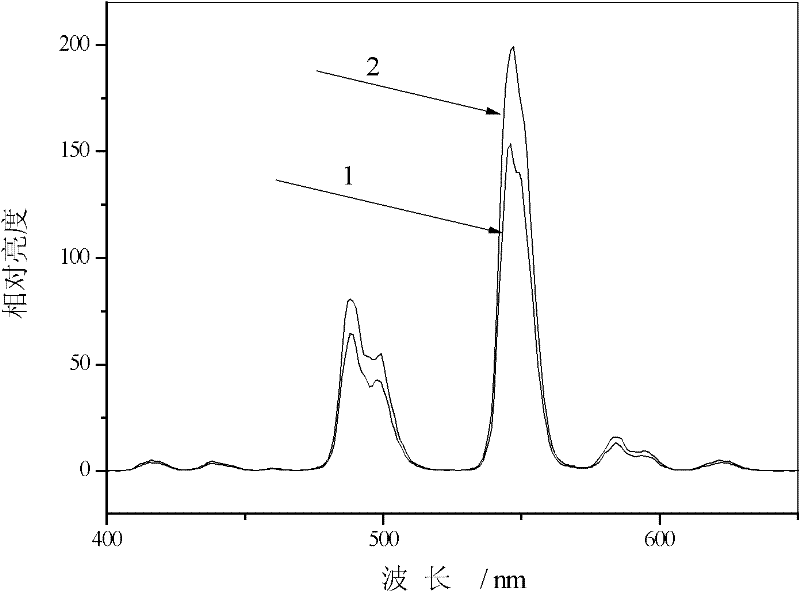
Yttrium Terbium silicate luminescent material and preparation method thereof
A technology of luminescent material and yttrium silicate, which is applied in the direction of luminescent materials, chemical instruments and methods, can solve the problems of weak luminescent properties of luminescent materials, and achieve the effects of improved luminescent properties, low equipment requirements, and simple processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
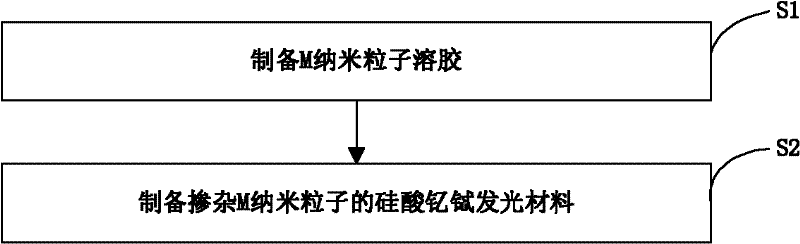
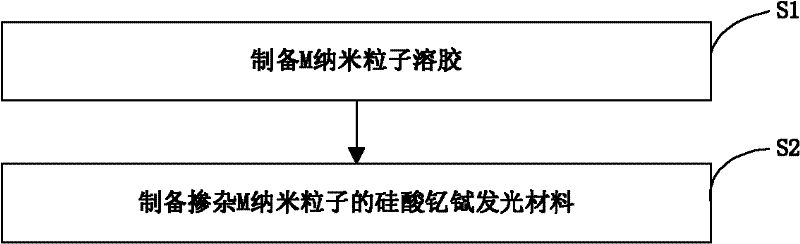
[0024] The preparation method of above-mentioned yttrium terbium silicate luminescent material, such as figure 1 As shown, the preparation process is as follows:
[0025] Preparation of step S1, M nanoparticle sol
[0026] 11), using water or ethanol as a solvent, taking a source compound containing M, such as silver nitrate, chloroauric acid, chloroplatinic acid, and palladium chloride as a solute, dissolved in the solvent to prepare an oxidizing agent solution; wherein, M is Ag , at least one of Au, Pt or Pd nanoparticles;
[0027] 12), using water or ethanol as a solvent, hydrazine hydrate, ascorbic acid, and sodium borohydride as a solute, that is, a reducing agent, is prepared into a reducing agent solution;
[0028] 13), under the state of magnetic stirring, dissolve the auxiliary agent into the oxidant solution in the above step 11), and make the content of the auxiliary agent in the finally obtained M nanoparticle sol be 5×10 -4 g / mL~4×10 -3 g / mL; Wherein, the auxi...
Embodiment 1
[0040] 1. Preparation of Pt nanoparticle sol
[0041] Weigh 5.18mg chloroplatinic acid (H 2 PtCl 6 ·6H 2 O) be dissolved in the deionized water of 15.2mL; After chloroplatinic acid dissolves completely, take by weighing 8.0mg sodium citrate and 12.0mg sodium dodecylsulfonate, and dissolve into chloroplatinic acid aqueous solution under the environment of magnetic stirring Medium; Weigh 3.8mg of sodium borohydride and dissolve it in 10mL of deionized water to obtain a concentration of 1×10 in 10mL -2 mol / L sodium borohydride aqueous solution; under the environment of magnetic stirring, according to the ratio of reducing agent to Pt ion molar weight ratio of 4.8:1, add 4.8mL sodium borohydride aqueous solution dropwise to chloroplatinic acid aqueous solution, and then continue the reaction 45min, that is, 20mL Pt content is 5×10 -4 mol / L Pt nanoparticle sol; then add 0.1g of PVP and stir for 14 hours to obtain surface-treated Pt nanoparticles.
[0042] 2. Preparation of yttri...
Embodiment 2
[0049] 1. Preparation of Ag nanoparticles sol
[0050] Weigh 17.0mg silver nitrate (AgNO 3 ) into 17.4mL of deionized water; when the silver nitrate was completely dissolved, weigh 60mg of sodium citrate, and dissolve it in an aqueous solution of silver nitrate under magnetic stirring; weigh 19mg of sodium borohydride and dissolve it in 10mL of deionized water , to obtain a 10mL concentration of 5×10 -2 mol / L sodium borohydride aqueous solution; under the environment of magnetic stirring, according to the ratio of reducing agent to Ag ion molar mass ratio of 1.3:1, add 2.6mL5×10 -2 mol / L sodium borohydride aqueous solution, and then continue to react for 30min to obtain 20mL silver content of 5×10 -3 mol / L Ag nanoparticle sol; then add 0.2g of PVP, and stir for 8h to obtain surface-treated Ag nanoparticles.
[0051] 2. Preparation of yttrium terbium silicate luminescent material doped with Ag nanoparticles
[0052] 1) Fully mix 2.0 mL of distilled water and 10 mL of absolu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com