Doxorubicin liposome and its preparation method
A kind of technology of doxorubicin lipid and liposome, which is applied in the field of medicine and achieves the effect of reasonable control of ethanol content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of doxorubicin hydrochloride liposomes (DOX-L) by pH gradient method
[0021] Preparation of blank liposomes
[0022] Weigh the lipid components, stir and dissolve them in a water bath at 65°C with an appropriate amount of absolute ethanol, evaporate the ethanol, add a citric acid-sodium citrate buffer solution with a certain concentration of pH=4.0, stir in a water bath at 65°C for 20 min, and obtain a blank lipid Primer. Sonicate the primary product probe for 8 min (200 w×2 min, 400 w×6 min), and pass through 0.8, 0.45, 0.22 μm microporous membranes in turn to obtain a blank liposome suspension (final phospholipid mass concentration 50 mg·mL -1 ).
[0023] Preparation and drug loading of gradient liposomes
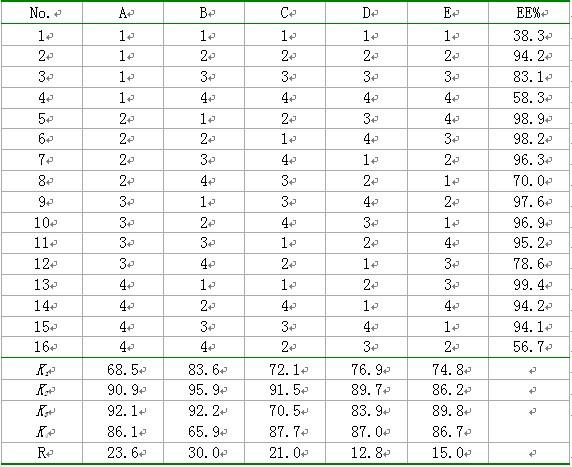
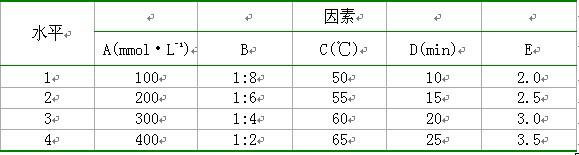
[0024] Take 1.0 mL of the above blank liposome suspension, add a certain amount of 500 mmol L -1 Sodium phosphate solution to obtain gradient liposomes. Take a certain amount of gradient liposome and doxorubicin hydrochloride solution...
Embodiment 2
[0039] Embodiment 2 The influence of ethanol residue on liposome
[0040] prescription composition
[0041] Distearoylphosphatidylcholine (DSPC) 3g
[0042] Cholesterol (CH) 1g
[0043] Macrogol 1000 Cholesterol Hemisuccinate (mPEG1000-CHEMS) 0.5g
[0044] Weigh the prescription quantities of DSPC, CH, mPEG1000-CHEMS, and use 2.5%, 5.0%, 10%, 15%, 20%, 25%, 30% and 40% (volume ratio of ethanol to hydration medium) of absolute ethanol respectively Dissolve the membrane material, add 300 mmol·L of pH=4.0 -1 citric acid-sodium citrate buffer solution, the final concentration of phospholipids was 50 mg·mL -1 , and stirred in a water bath at 65°C for 20 min to obtain the primary blank liposome. Sonicate the primary product probe for 8 min (200 w×2 min, 400 w×6 min), then pass through the microporous membranes of 0.8, 0.45, and 0.22 μm in sequence to obtain a blank liposome suspension, which was measured by laser particle size The particle size of each liposome was measured by...
Embodiment 3
[0046] Embodiment 3 Adriamycin liposome preparation
[0047] Preparation of lipid mixture powder: mix HSPC, CHOL and PEG2000-DSPE according to the mass ratio (3:1:1), dissolve in ethanol to obtain a clear solution, and obtain lipid powder after spray drying (tert-butanol can also be used to dissolve each Lipid composition, lipid powder obtained by freeze-drying method).
[0048] Preparation of blank liposomes: the above-mentioned lipid powder was hydrated with 300 mM ammonium sulfate solution at 50-65° C., and sheared and dispersed to obtain multilamellar liposomes with uneven particle sizes. Add different amounts of absolute ethanol, use microfluidic equipment to reduce the particle size of liposomes (about 80nm), and prepare liposomes containing different concentrations of ethanol.
[0049] Preparation of gradient liposomes: use ion exchange resin / fiber to remove ammonium sulfate in the outer water phase of liposomes.
[0050] Drug loading: add adriamycin hydrochloride sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com