Application of 1 beta-hydroxy alantolactone in preparation of medicine for preventing and curing rheumatoid arthritis
A technology of inulin and arthritis, which is applied in the application field of medicine, and can solve problems such as inflammation and sequelae treatment stage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of 1β-hydroxy inulin
[0021]10.0kg of dry inula (I.japonica) aerial parts (including stems, leaves and flowers collected from Haozhou, Anhui) were pulverized and extracted 3 times with 95% ethanol at room temperature (temperature 25° C.) 100L), each time is 24, 12, 12 hours respectively. After diluting 427.5 g of the obtained crude extract with an appropriate amount (10 L) of water, dilute with petroleum ether (divided into four times, each 40 L), dichloromethane (divided into four times, each 40 L), ethyl acetate (divided into four times, each 40 L) , each 40L), n-butanol extraction (four times, each 40L). 50.5 g of the dichloromethane extract obtained therein was separated with 200-300 mesh thin-layer silica gel, and dichloromethane-methanol (100:0, 50:1, 20:1, 10:1, 5:1, 2: 1,) carry out gradient elution. Thin-layer chromatography detection (2010 editions Chinese Pharmacopoeia Appendix VI B thin-layer chromatography), collects the component ...
Embodiment 21
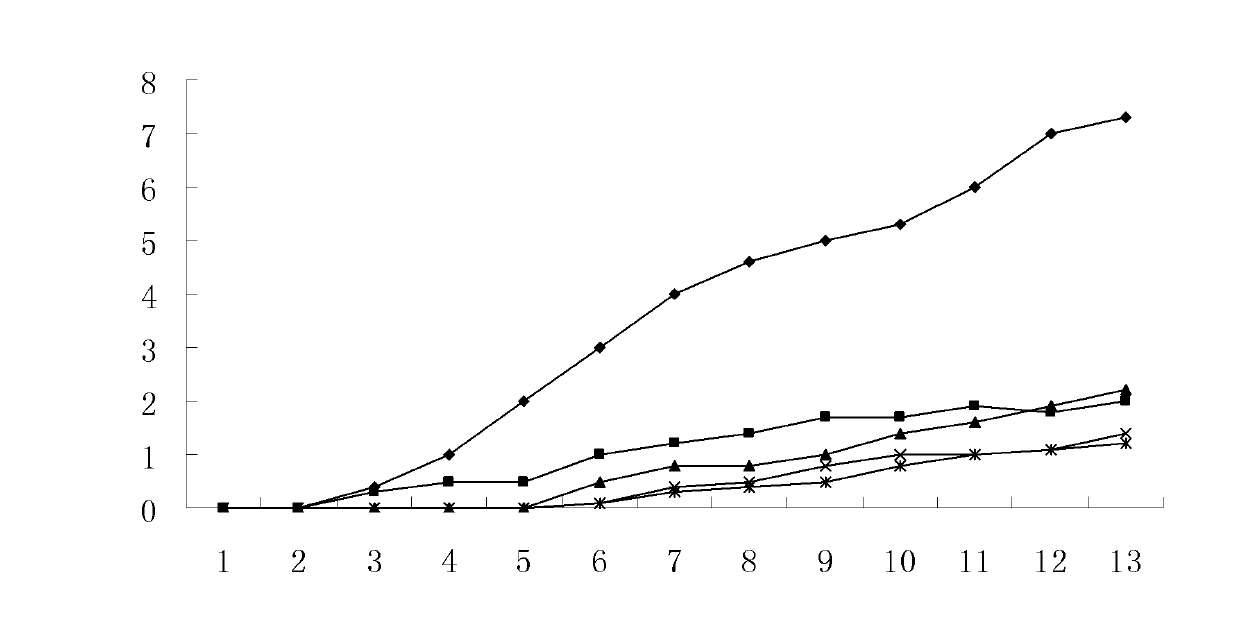
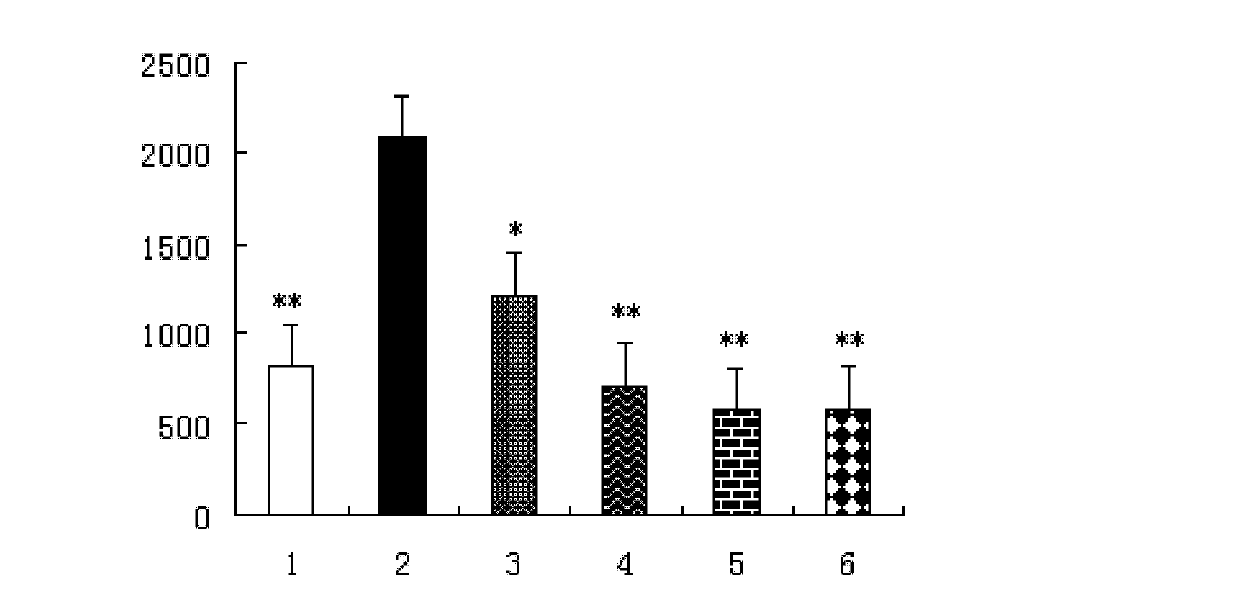
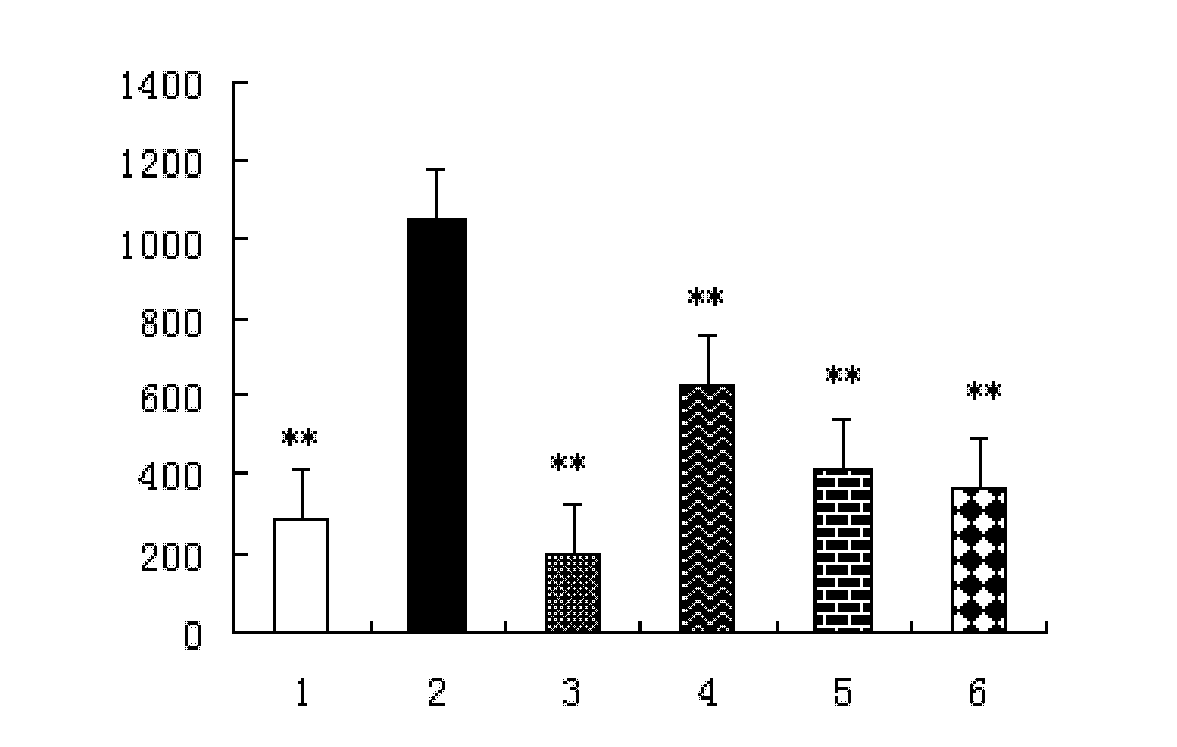
[0022] Example 21 Beta-Hydroxy Inulin Animal Drug Efficacy Test
[0023] 1. Experimental materials
[0024] Drugs and Reagents
[0025] 1β-hydroxy inulin: prepared in Example 1, dissolved in a 3‰ CMC-Na (sodium carboxymethylcellulose) solution to a 1 mg / ml solution. Different volumes were intraperitoneally injected according to the body weight of the animals.
[0026] Chicken type II collagen (C II): Chondrex, Redmond, WA 98052, USA;
[0027] Incomplete Freund's Adjuvant (IFA) Difco Laboratories, Detroit, MI;
[0028] Phosphate Buffered Saline (PBS): Prepared by our laboratory: Dissolve 8gNaCl, 0.2g KCl, 1.44g NaCl in 800ml distilled water 2 HPO 4 and 0.24g KH 2 PO 4 . Adjust the pH to 7.4 with hydrochloric acid, add water to make up to 1L, filter and sterilize before use;
[0029] DMSO dimethyl sulfoxide (Dimethyl sulfoxide, DMSO): a product of sigma;
[0030] 1640 Medium: Gino Biomedical Technology Co., Ltd.;
[0031] Calf serum: Minhai Biological Co., Ltd.;
[0...
Embodiment 3
[0066] tablet:
[0067] Preparation method: mix 1β-hydroxy inulin, lactose and starch, moisten evenly with water, sieve the wetted mixture and dry it, then sieve it, add magnesium stearate, then press the mixture into tablets, each tablet weighs 300mg, 1β-hydroxy inulin content is 25mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com