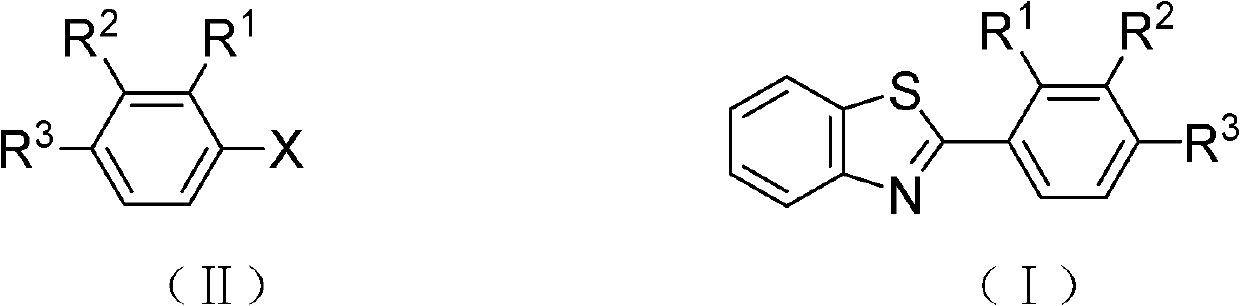
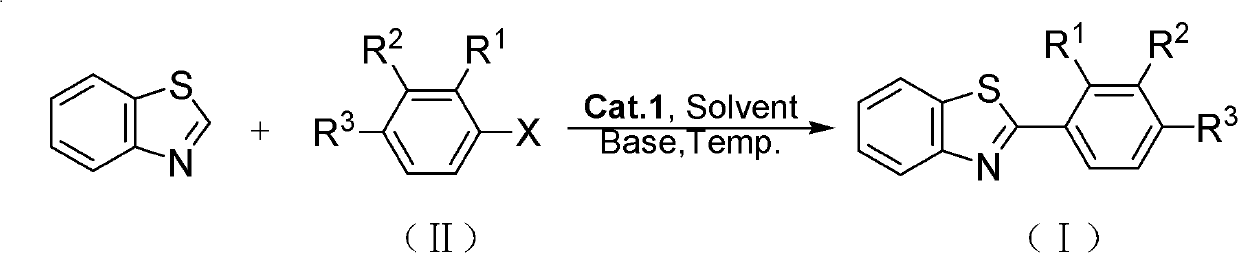
Preparation method of coupling aromatic compound
An aromatic compound and coupling technology, applied in the field of preparation of coupled aromatic compounds, can solve the problems of high toxicity, easy inactivation, limited industrial application, etc., and achieve the effects of mild reaction conditions and reduced use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 oxime ether cyclopalladium complex
[0033] Add p-hydroxyacetophenone oxime (1.43g, 9.5mmol), lithium tetrachloropalladate (2.62g, 10.0mmol), sodium acetate (0.82g, 10.0mmol) and 8mL methanol, magnetic Stirring was heated and refluxed for 72 hours, the filter cake was removed by filtration, and 10 mL of distilled water was slowly added dropwise to the filtrate to obtain 2.16 g of a yellow-green solid with a yield of 75%.
[0034]
Embodiment 2
[0036] Add benzothiazole (135.2mg, 1.0mmol), iodobenzene (306.0mg, 1.5mmol) to the two-necked reaction flask, the oxime ether cyclopalladium complex (14.7mg, 0.05mmol) prepared by the method in Example 1, K 2 CO 3 (276.0mg, 1.5mmol), DMAc (5mL), under argon atmosphere, heated to 140 ° C, refluxed for 24 hours, after the reaction, the reaction solution was distilled off the solvent under reduced pressure, and the concentrate (residue) was subjected to silica gel column chromatography Separation [ethyl acetate and petroleum ether (volume ratio: 1:50) as the eluent], the eluate was collected at Rf value = 0.32, rotary evaporated, and dried to obtain 2-phenylbenzothiazole (I-1) 169.0 mg, yield 80%, white solid, melting point: 86-87°C.
[0037]
Embodiment 3~18
[0039] The reaction conditions are as shown in Table 1, and other operations are the same as in Example 2.
[0040] Table 1 Screening of reaction conditions
[0041]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com